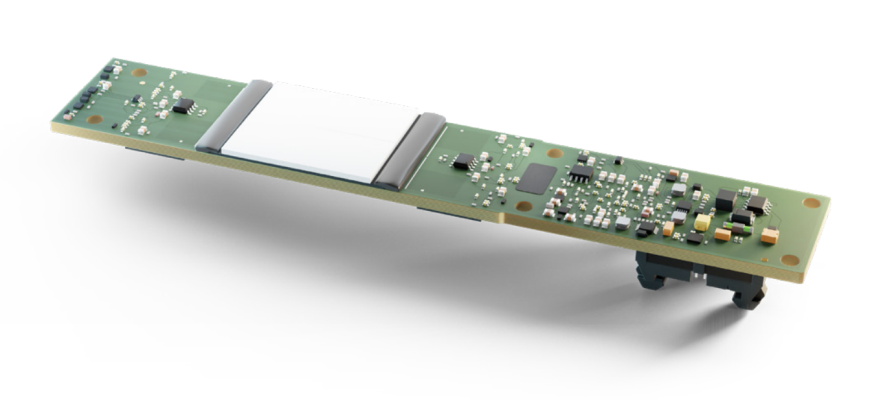
November 28, 2021 — Detection Technology, a leader in X-ray detector solutions, unveiled the industry’s first off-the-shelf detector series optimized for value and mainstream medical CT (computed tomography) imaging needs. The plug-and-play-type series, branded as X-ACE, is available as stand-alone detector boards, X-ACE 16, and X-ACE 32, and alternatively as complete subsystems that include ready-made detector boards, controllers, software libraries, and all the necessary accessories. The investment-free X-ACE series brings notable total cost savings and speeds up the time-to-market of advanced CT systems in the highly competitive medical imaging segments.
The X-ACE product family is built on the well-proven and modular platform. The platform covers a wide imaging area and features the highest level of integration, as a scintillator, a photodiode, analog-to-digital converters (ADC), and a field-programmable gate array (FPGA) are assembled to a single printed circuit board (PCB). This means simplified system designs, straightforward integration, minimized risks, a streamlined supply chain, and detector solutions that are mechanically more robust and digitally enhanced. The platform easily scales up from 16-slice to 32-slice system configurations to cover a wide range of set-ups and provides 20-millimeter coverage at the isocenter.
“Once again, we have set a new industry standard in medical CT imaging. We introduced the world’s first off-the-shelf, tileable CT detector module, named X-Tile, for the highest-tier volumetric CT systems in 2017. We rolled out our latest innovation in the field. The X-ACE completes our offering for medical CT applications. We are proud to state that our company is the only one in the market to enable one-stop shopping. We have available standard medical CT solutions for all medical CT imaging modalities, from the value to the premium segment,” said Tuomas Holma, director of product management in the Medical Business Unit.
“The X-ACE stands for all-in-one CT imaging experience as well as achieving cost effectiveness in this fast-paced, fiercely competitive marketplace. The key features are a perfect fit for the requirements of the target market. For example, the X-ACE 16 has already proven its importance in the value segment during the COVID-19 pandemic, when the market witnessed a renaissance of 16-slice X-ray imaging systems used for the diagnosis and treatment of COVID patients. This trend is here to stay and will intensify, especially in the emerging economies.”
The X-ACE provides high image quality with low doses at fast scanning speeds for enhanced patient safety and experience. State-of-the-art performance is achieved by a pixelated, ultra-fast ceramic scintillator, coupled with a high-performance frontside-illuminated (FSI) photodiode. The characteristics of the most sensitive and ultra-low dark current photodiode are made possible by utilizing Detection Technology’s proprietary FSI photodiode manufacturing process.
“We have a comprehensive track record as the most trusted provider of customized detector solutions for medical CT applications, and now we have positioned ourselves as a pioneer of the standard offering. Our off-the-shelf CT detector solutions enable rapid and cost-efficient system development yet meet the most stringent medical standards and regulation. For us, providing total cost savings does not mean sacrifices in image quality. By rethinking and simplifying detector designs, we have achieved win-win gains,” Holma said.
To facilitate and accelerate the system design phase and detector integration, Detection Technology provides global engineering support and a complete developer kit. Engineering samples of the ACE series are available, and inquiries about the developer kit can be directed to the company.
For more information: www.deetee.com


 February 16, 2026
February 16, 2026 









