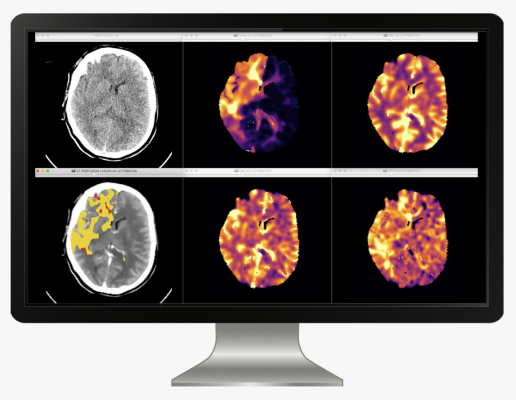
February 23, 2021 — icometrix, world leader in imaging artificial intelligence (AI) solutions for people with neurological conditions, announced the addition of icobrain cva, a stroke solution, to the icobrain portfolio. The announcement follows the FDA-clearance and CE-marking of its image processing software for the analysis and communication of the tissue perfusion state on computed tomography (CT) perfusion scans in patients with ischemic stroke. icobrain cva provides physicians with fast, fully automated, and state-of-the-art insights to support treatment decisions in acute ischemic stroke.
Since 2018, treatment guidelines for stroke have expanded the treatment window for mechanical thrombectomy from 6 to 24 hours. Treatment for ischemic stroke is not without risk and requires careful patient selection based on their tissue status. The automated assessment of tissue parameters in an acute clinical setting by icobrain cva will allow more patients to get the right treatment and can improve patient outcomes and care while increasing efficiency.
"With the launch of icobrain cva we address a persisting need in the treatment of acute ischemic stroke. By democratizing advanced CT perfusion analysis for healthcare systems worldwide we take the next step in our mission to become a holistic brain solution provider," said Wim Van Hecke, CEO at icometrix.
"The main challenge of current stroke solutions is correctly identifying the entry point of the injected contrast in the brain. icobrain cva introduces new, patented, deep learning technology into this identification process to achieve a more robust assessment of the infarcted area," explained Dirk Smeets, CTO at icometrix.
icobrain cva is a fully-automated CE-marked and FDA-cleared software solution for the quantitative assessment of tissue perfusion on CT. icobrain cva reports the volume of the core and perfusion lesion by quantifying reduced cerebral blood flow, volume, and transit time. The report includes information on the correctness of the selected arterial input function and the quality of the output. icobrain cva is a cloud-based solution, returning a report and perfusion maps straight into your PACS and e-mail inbox.
For more information: icometrix.com


 February 13, 2026
February 13, 2026 









