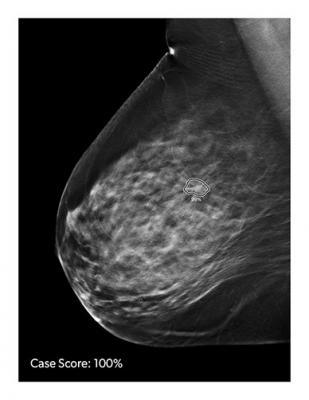
Image courtesy of iCAD
The Federal Food, Drug, and Cosmetic Act (FD&C Act), as amended, establishes a comprehensive system for the regulation of medical devices intended for human use. Section 513 of the FD&C Act (21 U.S.C. 360c) established three classes of devices, reflecting the regulatory controls needed to provide reasonable assurance of their safety and effectiveness. The three classes of devices are class I (general controls), class II (special controls), and class III (premarket approval).
Devices that were not in commercial distribution prior to May 28, 1976 (generally referred to as postamendments devices), are automatically classified by section 513(f)(1) of the FD&C Act into class III without any FDA rulemaking process. Those devices remain in class III and require premarket approval unless, and until, the device is reclassified into class I or II, or FDA issues an order finding the device to be substantially equivalent, in accordance with section 513(i) of the FD&C Act, to a predicate device that does not require premarket approval. The Agency determines whether new devices are substantially equivalent to predicate devices by means of premarket notification procedures in section 510(k) of the FD&C Act (21 U.S.C. 360(k)) and part 807 (21 CFR part 807).
A postamendments device that has been initially classified in class III under section 513(f)(1) of the FD&C Act may be reclassified into class I or II under section 513(f)(3). Section 513(f)(3) of the FD&C Act provides that FDA acting by order can reclassify the device into class I or II on its own initiative, or in response to a petition from the manufacturer or importer of the device. To change the classification of the device, the proposed new class must have sufficient regulatory controls to provide a reasonable assurance of the safety and effectiveness of the device for its intended use.
On June 4, 2018 (83 FR 25598), FDA published in the Federal Register a proposed order to reclassify the device type from class III to class II, subject to premarket notification. The comment period on the proposed order closed on August 3, 2018.
You can read the full published final order here.


 March 06, 2026
March 06, 2026 









