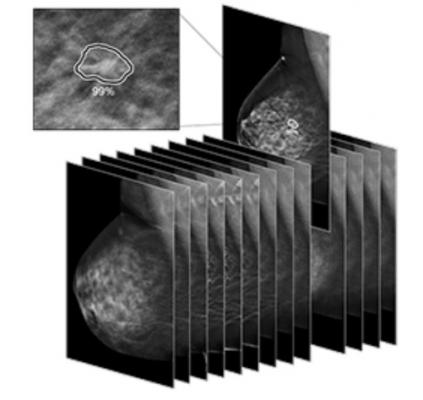
July 10, 2019 — Arizona-based SimonMed Imaging announced their implementation of the first U.S. Food and Drug Administration (FDA)-cleared artificial intelligence (AI) program for significantly enhancing early breast cancer detection for 3-D mammography. The AI program, called ProFound AI for digital breast tomosynthesis (DBT), is a technology developed by iCAD Inc.
ProFound AI for DBT is a high-performance, deep learning, workflow solution that assists radiologists reading mammography. It is the first and only FDA-cleared 3-D tomosynthesis technology using artificial intelligence, according to the company.
“Implementing ProFound AI into our processes for breast cancer screenings will improve detection of breast cancer and reduction of false positives and recalls,” said John Simon, M.D., founder and CEO of SimonMed. “With the enhanced technology available through 3-D mammography, the review of the 84 slices of breast tissue per breast will now be expedited, but does not replace the specialized radiologist.”
SimonMed Imaging is one of the largest outpatient medical imaging providers and largest physician radiology practices in the United States.
ProFound AI for DBT was FDA-cleared in 2018 and offers benefits to both radiologists and patients alike. Designed to detect malignant soft-tissue densities and calcifications, the software provides radiologists with key data, such as Certainty of Findings and Case Scores, which can assist radiologists in making clinical decisions and prioritizing caseloads. The technology has been clinically proven to improve cancer detection rates by 8 percent, reduce unnecessary patient recall rates by 7.2 percent, and slash radiologists’ reading time by 52.7 percent and up to 57.4 percent for dense breast 3-D image analysis.
For more information: www.icadmed.com


 March 06, 2026
March 06, 2026 









