Image courtesy of GE Healthcare
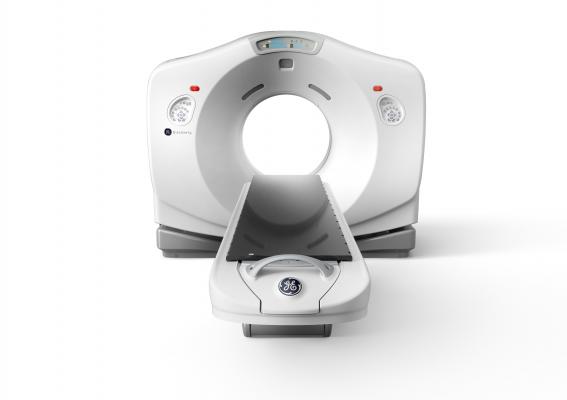
November 20, 2015 — GE Healthcare introduced the Discovery RT, a new advanced radiation therapy planning computed tomography (CT) system at the 57th annual American Society for Radiation Oncology (ASTRO) meeting in San Antonio, Texas, Oct. 18-21.
Discovery RT is a CT simulator that that enables flexible patient positioning, software to address challenges presented by patient motion and metal, precise treatment planning and an efficient workflow. CT simulation is used to duplicate the radiation-treatment machine in terms of its geometric, mechanical and optical properties by creating a three-dimensional image dataset. This data is then used by the clinicians to localize the tumor and plan treatment without physically having the patient present.
Discovery RT boasts an 80 cm bore with an 80 cm field-of-view, enabled by Max Field of View* (MaxFOV), a new feature of this system. MaxFOV delivers CT images with specified spatial and density values to the edge of the bore — an industry first, according to the company. The large bore and MaxFOV enables the flexibility to position the patients in different treatment positions and then to image the full patient volume for more accurate simulation and therapy planning.
GE Healthcare’s Smart Deviceless 4-D technology delivers workflow efficiency and patient comfort in 4-D motion assessment. Smart Deviceless 4-D provides and displays images of all phases of a breathing cycle, which helps to simplify the motion assessment workflow. Deviceless 4-D assesses motion without an external respiratory gating device, another unique feature of the Discovery RT system designed to improve workflow and patient comfort with no compromise to accuracy and precision.
The Discovery RT combines Smart Deviceless 4-D technology with Smart Metal Artifact Reduction (MAR) to address two of treatment planning’s biggest challenges – the ability to outsmart metal and motion. When utilizing CT scans, clinicians are often challenged by image distortions caused by high-z metals in the body such as hip prosthesis, screws or dental fillings. Metals often generate streak-like artifacts in CT scans, making it difficult to clearly delineate between tumors and healthy tissue. Smart MAR addresses this challenge with a three-stage, projection-based method designed to address both causes of metal artifacts – photon starvation and beam hardening. This automated approach is designed to reveal anatomic details obscured by metal artifacts.
The GE MR Radiation Oncology Suite is another addition to the complete portfolio of radiation oncology solutions from GE Healthcare. The suite includes a full complement of coils, positioning devices and pulse sequences to get voxels the right size in treatment position that can improve accuracy for treatment planning. In addition to the MR (magnetic resonance) oncology suite, the Signa PET/MR, which has been commercially available in the United States since November 2014, offers time-of-flight positron emission tomography (PET) reconstruction with high sensitivity to explore lower PET dose opportunities. The Signa PET/MR is available with MR Silenz, part of SilentScan, with zero TE capability. Zero TE allows development in the area of bone segmentation, which has potential uses for MR simulation.
GE Healthcare also offers ExAblate, an MR-guided focused ultrasound that delivers high intensity focused ultrasound that thermally ablates targeted tissue using MRI to identify and target tumors while providing temperature monitoring of the treated tissue in real time. Also on display at ASTRO, the Discovery PET/CT portfolio features advanced motion management and Q.Clear, an exclusive reconstruction engine that improves up to two times the quantitation accuracy (SUVmean) and image quality (SNR), aimed at a more accurate treatment response assessment.
Discovery RT and MaxFOV are not yet CE marked.
For more information: www.gehealthcare.com


 March 03, 2026
March 03, 2026 









