November 6, 2013 — Samsung Electronics America Inc. announced the U.S. commercial release of its first
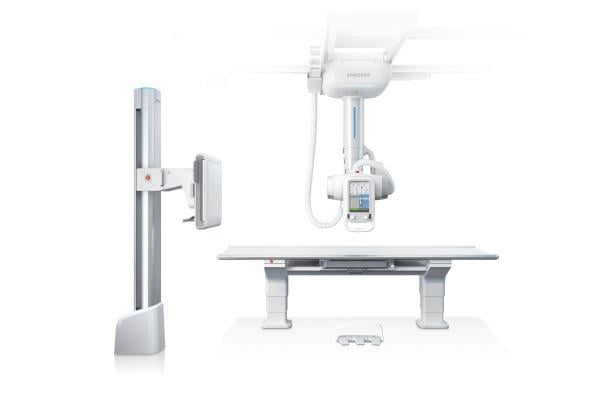
digital radiology (DR) system, the Samsung XGEO GC80. The Samsung XGEO GC80, which received 510(k) approval from the U.S. Food and Drug Administration (FDA) earlier this year, represents Samsung’s entrance into the DR market with a system offering advanced imaging technology, award-winning design and user-friendly workflows.
The XGEO GC80 provides a spectrum of diagnostic and patient-centric tools aligned to meet the various demands and needs of both care providers and patients. From workflow to dose management, Samsung’s digital
X-ray technology balances science with purpose without jeopardizing image quality.
Utilizing a Samsung TFT-based flat panel detector, the XGEO GC80 system incorporates proprietary Adaptive Local Contrast Stretching (ALCOS) software with automatic, customizable post-image processing for optimizing contrast and edge sharpness. This enables the system to deliver high image quality, fast results and provide diagnostic confidence across diverse applications.
The award-winning ergonomic design features complement the Samsung XGEO GC80’s advanced image quality. Integrating Samsung’s robotics technology, the soft handling function allows users to reposition the tube head unit (THU) with an easy movement for improved workflow and reduced fatigue. With dedicated automatic features, including positioning, tracking and parking, the XGEO GC80 combines modern efficiency with user convenience and patient safety. Audio/visual indicators highlight each procedural step, helping to simplify and enhance patient/provider interactions.