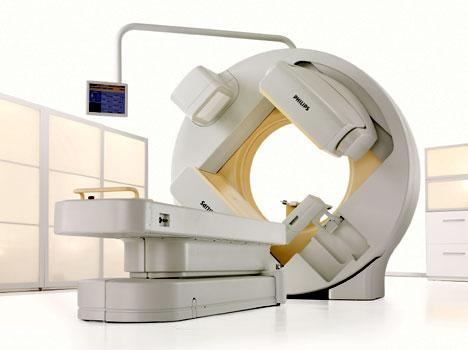
June 7, 2010 – Low-dose, flat-panel computed tomography (CT) iterative reconstruction was added to the BrightView XCT single photon emission computed tomography (SPECT)/CT system. The Full Iterative Technology (FIT) feature improves localization through better uniformity and less noise, leading to improvements in soft tissue image quality.
Philips Healthcare highlighted the technology today at the Society of Nuclear Medicine (SNM) annual meeting.
Already outfitted with the Philips Astonish advanced reconstruction algorithm, the new FIT offers a CT platform designed specifically for nuclear medicine. BrightView XCT Astonish image reconstruction technology provides significant spatial resolution improvement in cardiac SPECT studies compared to SPECT studies using filtered back projection. With FIT and Astonish, the system enables low patient X-ray dose levels, high-resolution localization and high-quality attenuation correction with the potential for fewer artifacts and shorter exam times. Philips will highlight new clinical evidence that Philips’ Astonish algorithms for nuclear cardiology help clinicians improve their diagnostic accuracy and increase their diagnostic confidence.
The BrightView XCT has a wide-open gantry and large bore that contributes to a positive patient experience. It offers a flat panel X-ray detector to be used for CT imaging in nuclear medicine. It is a compact unit that fits in the same size room as a small SPECT camera.
Philips is also showing its Concurrent Imaging technology for its SPECT systems, including BrightView and BrightView XCT. Concurrent technology makes image acquisition quicker, makes it easier to work with and evaluate images, and makes it possible to conduct multi-isotope evaluations simultaneously.
For more information: www.philips.com


 February 06, 2026
February 06, 2026 









