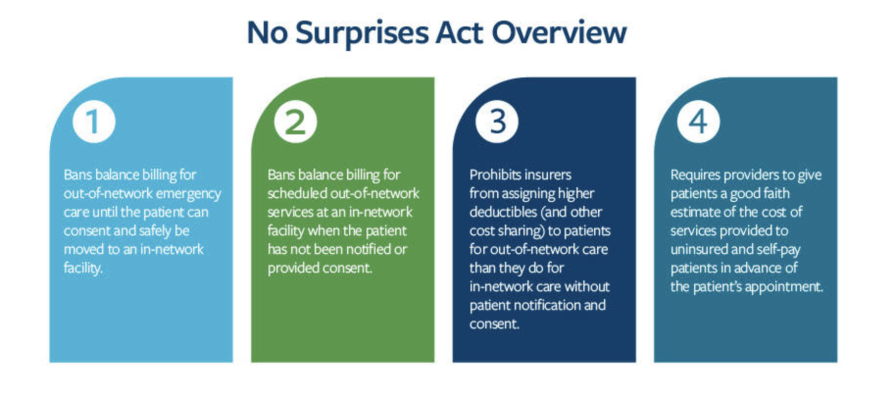
Graphic courtesy of The American College of Radiology
September 6, 2022 — On Aug. 19, the Departments of Labor, Health and Human Services (HHS), and the Treasury (“the Departments”) issued the Requirements Related to Surprise Billing: Final Rules. This set of rules is narrow in scope and only addresses the payment determination standards under the Federal independent dispute resolution (IDR) process and “downcoding” of claims by insurers. The American College of Radiology (ACR) has developed a detailed summary of the final rules.
The final rules direct certified IDR entities to select the offer that the IDR entity determines “best represents the value of the qualified item or service as the out-of-network rate.” The rules indicate that the qualifying payment amount (QPA) or median in-network rate is no longer presumed to be the most appropriate out-of-network rate. The rules then state that the IDR entity must first consider the QPA for the same or similar item or service and then must consider additional “credible” information submitted. IDR entities must provide explanations for every decision. However, if the IDR entity selects an amount that is not the QPA, it must explain what other credible information was considered and why those factors are not already accounted for in the QPA calculation.
The final rules also address “downcoding,” defined as “the alteration by a plan or issuer of a service code to another service code, or the alteration, addition, or removal by a plan or issuer of a modifier, if the changed code or modifier is associated with a lower QPA than the service code or modifier billed by the provider, facility or provider of air ambulance services.” The rules require insurers that downcode claims to provide a statement that the service code or modifier billed by the provider, facility or provider of air ambulance services was downcoded; an explanation of why the claim was downcoded, including a description of which service codes were altered, if any, and which modifiers were altered, added or removed, if any; and the amount that would have been the QPA had the service code or modifier not been downcoded.
The Consolidated Appropriations Act, 2021 (CAA), including the No Surprises Act (NSA), was enacted on Dec. 27, 2020. The NSA provides federal protections against surprise billing and limits out-of-network cost sharing under many of the circumstances in which unexpected bills arise most frequently. “Surprise billing” occurs when an individual receives an unexpected medical bill from a healthcare provider or facility after receiving medical services from a provider or facility that, usually unknown to the participant, beneficiary or enrollee, is a nonparticipating provider or facility with respect to the individual’s coverage.
For more information: www.acr.org
Related content:
CMS Withdraws Guidance on Surprise Billing, Out-of-Network Payment Disputes
ACR Joins "No Surprises Act" Lawsuit to Protect Patient Care
Major Medical Associations Ask Federal Court for Summary Judgement in No Surprises Lawsuit
Radiation Oncologists Applaud Biden-Harris Administration's Renewed Commitment to Cancer Moonshot
Racial/Ethnic Disparities Persist in Lung Cancer Screening Eligibility
Primary Lung Cancers Detected by LDCT are at Lower Risk of Brain Metastases
Physician and Patient Groups Call On CMS to Update Medicare Lung Cancer Screening Coverage
USPSTF Expands Lung Cancer Screening Eligibility Thresholds
Low-dose CT for Lung Cancer Screening: Benefit Outweighs Potential Harm
Physician and Patient Groups Call On CMS to Update Medicare Lung Cancer Screening Coverage


 March 06, 2026
March 06, 2026 









