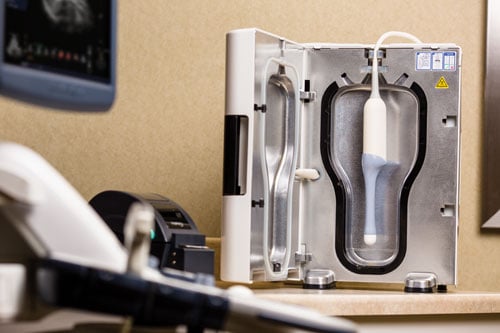
As demand for ultrasound rises, staff at St. Luke’s must make the most of every minute. The HLD system they use can save eight minutes per disinfection.
Manually soaking ultrasound probes has long been the standard. But ultrasound users and administrators know well the challenges that come with it. New technologies are helping to overcome them, offering quick, easy and cost-effective methods of high-level disinfection (HLD) to improve patient safety, staff efficiency and audit compliance.
Among the challenges faced by these technologies is compliance with The Joint Commission (TJC), whose auditors look not only at policies for probe disinfection, but at how well staff follow those policies. Soaking practices may vary, even among staff at the same institutions. Automated HLD systems, however, are programmed to follow the same routine probe after probe, offering the consistency that TJC auditors look for.
Another challenge is the impact that probe reprocessing has on workflow. Reprocessing involves three steps — pre-cleaning, disinfection and storage — and can be especially resource-intensive. Manual soaking requires one or more rooms typically outfitted with air exchangers to vent toxic fumes; eye wash stations installed nearby for use in case of accidents; and personal protective equipment worn by staff during the soaking process.
A core challenge is the efficacy of the process itself, which must kill the bacteria, viruses and fungi that threaten to cross-contaminate patients. Of special concern is the human papilloma virus (HPV), which is prevalent on probes after endovaginal exams and can resist disinfection by manual soaking.1-4
Seeking Compliance
Healthcare providers around the United States are already benefiting from automated HLD systems that address these challenges. At St. Luke’s University Hospital and Health Network in Bethlehem, Pa., it was the need for compliance that ultimately pushed key personnel to replace its long-standing manual soaking process for ultrasound probes with automated disinfection systems. It happened in 2013, when TJC arrived at St. Luke’s for a scheduled audit. “At St. Luke’s it is our utmost priority to be in compliance with soaking policies, so our desire to replace the long-standing manual soaking process for ultrasound probes with automated systems stemmed from that,” said Lindsey Solovey, director of imaging services for St. Luke’s, who was ultrasound manager at the time.
Froedtert Health and Medical College of Wisconsin in Milwaukee ran into similar trouble. “The Joint Commission came in and said, ‘Hey, where are your air exchangers? Where is your vented hood?’ ” said Darcy Lemke, Froedtert’s ultrasound manager.
Soon after, both St. Luke’s and Froedtert decided to switch from a manual soaking processes to the trophon EPR system, an automated technology that performs HLD within an enclosed chamber.
St. Luke’s has since installed about 20 of these systems, positioning them strategically throughout its half-dozen hospitals, multiple outpatient centers, labor and delivery rooms, and emergency rooms. More are planned, according to Solovey.
Froedtert now operates nine automated HLD systems at its two hospitals and several outpatient clinics. It is planning to install another five, with the goal of eliminating soaking entirely, according to Lemke.
See a 360 view of an ultrasound room with a trophon system installed.
Automated HLD
In its May 2014 bulletin “Quick Safety Alert,” TJC said its surveyors were increasingly finding noncompliance regarding the sterilization or high-level disinfection of medical equipment. By automating and documenting the disinfection process, automated HLD systems can ensure compliance for facilities, as well as improvements in patient safety and staff efficiencies. These systems streamline workflow, providing more time for caregivers to spend with patients while preventing the bottlenecks that can sideline staff. They are also friendlier to the environment and personnel, minimizing exposure to potentially hazardous chemicals otherwise used in the manual soaking process.
Going with automated systems in place of manual soaking, Solovey said, “is a no-brainer.”
The type of HLD device in use at St. Luke’s and Froedtert sprays a sonicated hydrogen peroxide mist on probes and handles, which hang suspended by their cables in enclosed chambers. After the disinfection cycle, any remaining hydrogen peroxide is chemically degraded into water and oxygen. A sensor indicates when the process is complete, signaling staff to open the door and remove the probe. A dime-sized, chemically treated cardboard disk changes from red to yellow when disinfection is successful.
Just as the use of sonicated hydrogen peroxide in HLD systems promotes patient safety by helping to reduce the risk of infections, so does it ensure compliance with many guidelines and standards. Automation is the key. When TJC found St. Luke’s noncompliant three years ago, “it was a department that didn’t use ultrasound frequently,” Solovey said. Auditors had found that the staff did not comply with the hospital’s own policy on how to manually disinfect the probes.
The automated process now in use at St. Luke’s is “dummy proof,” she said. “You cannot mess it up — it is impossible because of the automation.”
The trophon EPR requires no special equipment or room design. Mounted on walls or placed on countertops, it can be located in or near exam rooms. Alternatively, it can be transported to the point of care on mobile carts. If installed to replace the manual soaking process, centralized space previously dedicated to reprocessing can be repurposed, and air ventilation systems can be removed. The benefits of trophon HLD are multifold, including:
Faster, safer, friendlier. The manual soaking process is labor-intensive and time-consuming. At a minimum, it can take 20 minutes to complete. This includes cleaning and transporting the probe to a centralized room; putting on protective gear such as goggles, gowns and gloves; testing the soaking solution; then finally soaking the transducer. The process must also then be documented.
Automated HLD systems, such as the trophon EPR, are faster, safer and more user-friendly. With trophon EPR, the time from disinfection to storage is about 10 minutes — at least 10 minutes shorter than manual soaking. Staff are not required to don full protective gear, but rather wear only gloves. And because the disinfection process is completely enclosed, no fumes can escape to be breathed in by staff. Any hydrogen peroxide vapor that remains after the cycle finishes is chemically decomposed into harmless water and oxygen molecules, thereby protecting the environment and workers. Keeping disinfectants away from staff is important, as respiratory issues have been associated with use of disinfectants including formaldehyde, glutaraldehyde and chlorine, according to the Centers for Disease Control and Prevention.
Solovey said students who work at St. Luke’s comment on how they prefer the automated process to the manual soaking they must do at other facilities. This makes recruiting graduate sonographers easier, she said. It also makes hired staff more effective.
Increased effectiveness. When using an automated HLD system, staff can multitask, confident that probes will not be exposed to chemicals any longer than necessary. This is not so when disinfecting manually. Probes may soak for extended times because staff “get caught up in doing something else,” Solovey said. Distractions commonly occur in busy departments, according to Froedtert’s Lemke, who noted that probes have been left accidentally in chemical tubs overnight. Excessive exposure to disinfectants can turn otherwise-white probes a bluish-blackish color, according to Solovey. Noticing the discoloration, some patients have in the past questioned the cleanliness of probes, she said.
More time for patients. As demand for ultrasound rises, staff at St. Luke’s must make the most of every minute, according to Solovey. The HLD system they use can save eight minutes per disinfection. “That is a lot of time back into your day when you are doing 20 or 30 transvaginal ultrasounds daily, as some departments are,” she said. Eliminating the transport of probes to and from a centralized soaking station also saves time and can reduce chances of probe damage.
Simpler and faster disinfection resolves the bottlenecks that can occur when manually disinfecting probes. Pushed by a heavy volume of patients, staff may be tempted to cut corners, putting patients at risk of infection and the healthcare service in danger of being found noncompliant during an audit.
Documentation. Automated HLD systems can help improve compliance with healthcare recommendations and guidelines as they pave the way to a successful audit, providing auditors with the documentation they want to see. After each cycle, the trophon EPR systems at St. Luke’s and Froedtert print labels with the patient’s name, date and time, and whether the disinfection was successful. Staff then paste the label on a log sheet and initial it. This provides traceability in the event of an infection, as well as documentation for use during audits. Log sheets may be kept for several months; Froedtert keeps them for a half year. There is also the capability to trace each cycle log via software.
Offsetting Costs
Departments considering the switch to automated HLD systems must keep in mind the capital cost, according to Solovey and Lemke. When justifying the proposed purchase of HLD machines at St. Luke’s and Froedtert, they argued successfully that patient safety, increased compliance, efficiency and cost effectiveness outweighed the capital costs.
To bolster her case to key committees at St. Luke’s, Solovey prepared a cost-benefit analysis. Costs for disinfectant, neutralizer, air exchange filters and test strips were estimated at $65,000 annually. Consumables for automated HLD systems were calculated to cost about half as much. The analysis tipped further in favor of automated systems when reduced training and monitoring costs were figured in.
In her analysis, Solovey noted that St. Luke’s return on investment would take time. She estimated that capital costs would be offset partially by capital savings achieved by extending the lifespan of ultrasound probes, which may be reduced by overexposure to disinfectants, and by avoiding the need to buy additional probes to work around bottlenecks that may occur due to a centralized soaking process.
To minimize budget impact, St. Luke’s high-volume ultrasound departments were prioritized. By combining their HLD system purchases with other capital purchases, they were able to minimize budget impact. Froedtert and the Medical College of Wisconsin decided to replace chemical disinfectants in all departments and locations with trophon units as part of their capital project this year.
The Need For HLD
When deciding to switch to automated HLD systems, patient and staff safety were key considerations, Solovey and Lemke agree. Guidelines approved in April 2014 by the American Institute of Ultrasound in Medicine describe the use of a hydrogen peroxide nanodroplet emulsion in automated systems as providing “an effective high-level disinfectant without toxicity (to staff),” particularly as it pertains to clostridium difficile and the safe and effective disinfection of ultrasound probes.
Meta-analysis of peer-reviewed studies have found that low-level disinfection of endovaginal/rectal probes may leave 12.9 percent of transducers contaminated with pathogenic bacteria and 1 percent with frequently occurring viruses, namely HPV, herpes simplex virus and cytomegalovirus.5
Ultrasound probes are a potential source of HPV, which is among the most common sexually transmitted diseases and often inhabits transvaginal probes. A study conducted in Hong Kong and published in 2012 found that 7.5 percent of the probes used in the emergency room had DNA from HPV.1 The virus also resists glutaraldehyde and ortho-phthalaldehyde, two of the most common disinfectants used in manual soaking.2
Trophon EPR, which utilizes sonicated hydrogen peroxide, is currently the only system proven effective in killing HPV, according to research scheduled for publication in the American Journal of Obstetrics and Gynecology and available online since March 2016.3 In that study, the authors recommended the use of a sonicated hydrogen peroxide disinfectant system rather than aldehyde-type disinfectants.
In addition, research presented at the 2015 meeting of the Society of Healthcare Epidemiology of America showed that the use of nebulized 35 percent hydrogen peroxide in an automated system “is able to fully inactivate HPV, when used according to manufacturer instructions.” The researchers concluded later that year in the Journal of Medical Virology that “sonicated hydrogen peroxide offers an effective disinfection solution for ultrasound probes. Disinfection methods that are effective against HPV should be adopted where possible.”4
References:
Sponsored by GE Healthcare




 February 05, 2026
February 05, 2026 









