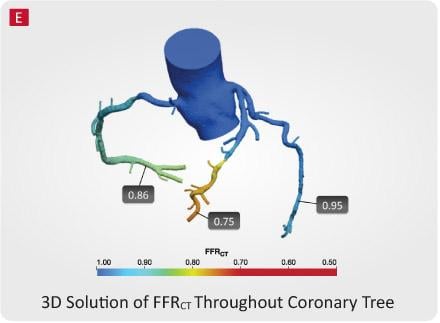
Image courtesy of HeartFlow Inc.
January 14, 2015 — University Hospitals (UH) Case Medical is now offering a new, non-invasive test for coronary artery disease designed to help physicians develop the right treatment plan for each patient. Developed by HeartFlow Inc., computed tomography-fractional flow reserve (CT-FFR) is a non-invasive imaging technology specifically designed to offer insight on both the extent of the blockage, as well as whether it is impacting blood flow to the heart. UH Case Medical Center’s Daniel Simon, M.D., will be first to use the CT-FFR test in the United States.
Most other diagnostic tests are designed to provide information to clinicians regarding a patient’s overall risk of having coronary artery disease, but they cannot help the clinician determine the extent to which a specific blockage is impeding blood flow to the heart.
The CT-FFR platform was developed by marrying non-invasive 3-D imaging with computational fluid dynamics technology to produce detailed models of a patient’s cardiovascular anatomy. For every patient, CT-FFR performs millions of complex equations simulating blood flow in the coronary arteries to provide mathematically computed FFR values from images derived using non-invasive CT angiography. Fractional flow reserve values demonstrate blood pressure differences around a lesion to determine whether it is likely to reduce blood flow to the heart. These simulated values help physicians determine the right course of action for each patient.
“CT-FFR represents a tremendous advancement in the diagnosis of coronary artery disease,” said Simon, president, Harrington Heart & Vascular Institute at UH Case Medical Center and Herman K. Hellerstein Chair and professor of medicine at Case Western Reserve University School of Medicine. “Historically, we have been faced with either using tests we knew were not always accurate or putting a patient through an invasive procedure just to determine whether they needed another invasive procedure. For the first time, we have access to a diagnostic that is both non-invasive and highly accurate in showing us the extent of a lesion, as well as how it can hinder blood flow through the vessel. I believe CT-FFR has the potential to completely change the way we manage coronary artery disease globally.”
Clinical data from the HeartFlow NXT study demonstrated superior discriminatory ability to identify lesions that have the potential to impede blood flow when compared to coronary CT angiography alone. In the study, published in the Journal of the American College of Cardiology in 2014, CT-FFR had higher diagnostic accuracy (86 percent) than coronary CT angiography (65 percent). This difference is primarily due to a significantly increased specificity with CT-FFR (86 percent) compared to coronary CT angiography (60 percent). Invasive angiography performed with 71 percent accuracy in the study.
With the improved data, patients will not have to undergo unnecessary procedures or further invasive or time-consuming testing associated with coronary artery disease.
For more information: www.heartflow.com


 February 09, 2026
February 09, 2026