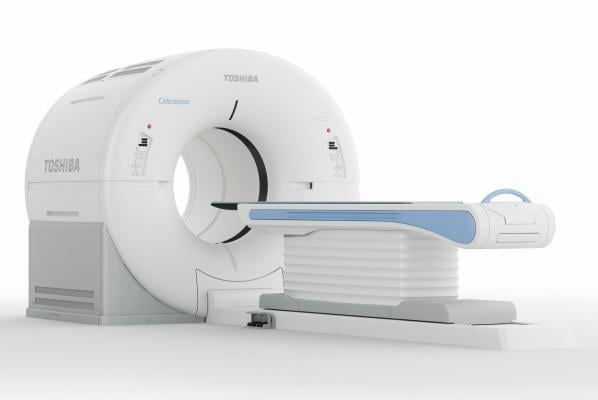
Toshiba Medical launches its new Celesteion PUREViSION Edition PET/CT system to help diagnose and treat oncology patients.
Oncologists have access to advanced imaging technologies for excellent cancer patient care with the new Celesteion PUREViSION Edition PET/CT system from Toshiba Medical, a Canon Group company. The newest edition of Celesteion allows for ease and efficiency in PET/CT, CT simulation and diagnostic CT exams, to help diagnose and treat oncology patients. The system’s patient-centered design can help deliver a safe and comfortable experience, facilitating better patient compliance.
The Celesteion PUREViSION Edition presents a significant advancement in PET/CT resolution and reconstruction. Offering 70 cm true field-of-view, as well as Toshiba Medical’s SUREFLiGHT reconstruction technology, including Point Spread Function and Time of Flight techniques, the system offers oncologists sharp images and high contrast for improved visualization of small tumors throughout the body. The system’s PUREViSION 16-row CT detector assists in acquiring high-quality CT images, while SEMARTM helps reduce artifacts caused by metal implants, allowing providers to view more clinical information.
Prioritizing patient comfort, the Celesteion PUREViSION Edition features an industry-leading 90 cm wide CT bore that creates a sense of openness to put patients at ease and offers versatile positioning for optimal treatment planning. Ensuring clinicians don’t have to choose between efficiency and safety, the system also comes standard with AIDR 3D iterative dose reduction technology.
“We developed the Celesteion PUREViSION Edition with our customers’ needs in mind,” said Dominic Smith, senior director, CT, PET/CT, and MR Business Units, Toshiba America Medical Systems, Inc. “Accuracy is everything when treating oncology patients and Toshiba Medical is committed to providing our customers with the high-quality imaging solutions they require to provide efficient, effective patient care.”
Toshiba Medical will showcase the Celesteion PUREViSION Edition at this year’s Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in Denver, June 10–14, 2017.
For more information: www.medical.toshiba.com


 January 22, 2026
January 22, 2026