September 18, 2017 — Toshiba Medical announced that it will display several new enhancements to its existing computed tomography (CT) technology at the 2017 Radiological Society of North America (RSNA) annual meeting, Nov. 26-Dec. 1 in Chicago.
The company is introducing enhancements to the Aquilion One Genesis Edition for fast and safe CT imaging in the emergency department. The upgraded premium CT system now offers Toshiba Medical’s FIRST (MBIR) capability for low-contrast detectability in the brain while opening doors for clinicians to possibly see early signs of stroke with CT. The system can help improve workflow for advanced CT angiography (CTA) scans while potentially reducing IV contrast and radiation dose with three-phase Variable Helical Pitch (vHP3), which automatically changes from an electrocardiogram (ECG)-gated to a non-ECG-gated acquisition during a single helical scan. The Aquilion One Genesis Edition also features an optimized beam spectrum based on PUREViSION Optics, combined with PUREViSION CT Detector and FIRST (MBIR) reconstruction, offering superior image detail and resolution and up to 82.4 percent dose reduction, according to Toshiba. Additionally, quick scans are made possible with SUREPosition, which accurately centers the patient within the gantry without clinicians having to manually adjust the patient.
The Aquilion Lightning 80 is a total cost-of-ownership solution with high-end CT technology. The system delivers fast reconstruction speeds of up to 50 images per second at full resolution, and with slices at 0.5 mm and a 78 cm bore, it optimizes workflow and patient comfort. To simplify exams, the Aquilion Lightning 80 comes standard with Adaptive Diagnostic solutions, such as Single Energy Metal Artifact Reduction (SEMAR) and SURESubtraction. The system can also be reconfigured from 40 to 80 to 160 slices (with coneXact) without replacing hardware. Design features of Aquilion Lightning 80 make this scanner a wide, fast and small solution for healthcare providers.
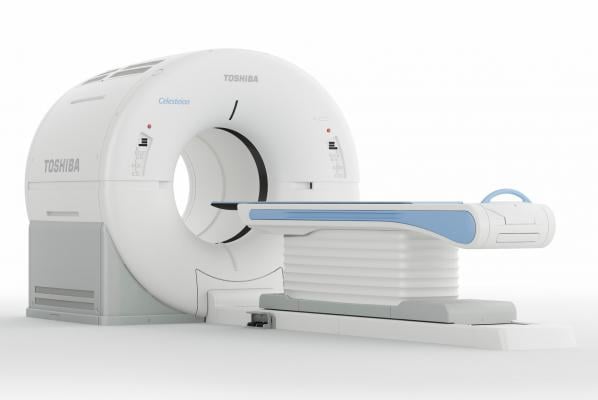
Finally, the company will display the Celesteion PureViSION Edition positron emission tomography (PET)/CT system that provides oncologists access to advanced nuclear imaging technologies for cancer patient care. The newest edition of Celesteion offers 70 cm true field-of-view, as well as Toshiba Medical’s SUREFLiGHT reconstruction technology, including Point Spread Function and Time of Flight techniques, providing oncologists with sharp images and high contrast for improved visualization of small tumors throughout the body. The system’s PUREViSION 16-row CT detector assists in acquiring high-quality CT images, while SEMAR helps reduce artifacts caused by metal implants. Prioritizing patient comfort and safety, the Celesteion PUREViSION Edition features a 90 cm wide CT bore and comes standard with AIDR 3-D iterative dose reduction technology.
For more information: www.medical.toshiba.com


 March 03, 2026
March 03, 2026 









