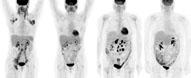
June 16, 2009 - The SNM 2009 Image of the Year is an image depicting how radioimmunotherapy can successfully treat a potentially fatal type of lymphoma, which was presented at SNM’s 56th Annual Meeting June 13–17, 2009, in Toronto.
Radioimmunotherapy is a form of personalized medicine that combines the cancer-fighting ability of radiation therapy with the precise targeting capacity of immunotherapy. This year’s winning image comes from a study that examines two radioimmunotherapy agents and their effectiveness in treating non-Hodgkin’s lymphoma (NHL). The image is actually two sets of before-and-after PET scans of two patients. One patient was treated with Iodine-131 tositumomab (Bexxar). The other received Yttrium-90 ibritumomab tiuxetan (Zevalin). The PET images reveal that both patients showed no metabolically active NHL as early as three months after treatment.
“This image is really remarkable because it shows two positive benefits of molecular imaging and nuclear medicine at the same time,” said Henry N. Wagner Jr., SNM past president and professor at Johns Hopkins University Bloomberg School of Public Health, who annually selects the SNM Image of the Year from thousands presented at the Annual Meeting. “First, the PET scans demonstrate the power of radioimmunotherapy to fight advanced cases of non-Hodgkin’s lymphoma. In addition, this is proof of how PET scans are indispensable tools for managing patient care and determining whether treatments are working as intended.”
Each year, more than 66,000 new cases of non-Hodgkin’s lymphoma are diagnosed in the United States, and more than 19,000 patients die of the disease annually. About 85 percent of non-Hodgkin lymphomas in adults are B-cell in origin. Currently, Bexxar and Zevalin are approved by the FDA for the treatment of patients with CD20 antigen-expressing relapsed or refractory, low-grade, follicular or transformed non-Hodgkin’s lymphoma, including patients with Rituximab-refractory non-Hodgkin’s lymphoma.
“Our study shows that both of these radioimmunotherapy agents are safe and effective in treating NHL, even for patients with extensive disease,” said Andrei Iagaru, instructor of nuclear medicine in the Department of Radiology at Stanford University Medical School, Stanford, Calif., and lead author of the study. “In fact, our study found that as many as 70 percent of patients had objective responses to the radioimmunotherapy, and about one third of the patients showed complete responses.”
The study followed 71 patients with relapsed or refractory non-Hodgkin’s lymphomas who underwent treatment with either Bexxar or Zevalin. The results indicate that both drugs are safe and effective. Of the group of patients who received Bexxar, 24 of 35 patients responded to the drug. Of the group who received Zevalin, 28 of 36 responded to the treatment. Between the two groups of patients, 27 showed complete response to the drugs; however, not all patients in the study were helped by the radiopharmaceuticals. In 19 of the 71 patients, disease stayed the same or progressed despite the treatment.
“One question that a lot of researchers are asking is whether these results would be even better if NHL patients were allowed to receive radioimmunotherapy as a first-line treatment instead of having to wait until other therapies fail,” said Iagaru. “The patients in our study were all in advanced stages of disease. If they had received radioimmunotherapy treatment earlier, it’s possible that their responses would have been even stronger. This is the focus of future research projects.”
Scientific Poster 47, A. Iagaru; E. Mittra, M. Goris, Division of Nuclear Medicine, Stanford University Medical Center, Calif.,“131I-tositumomab (Bexxar) vs. 90Y-ibritumomab (Zevalin) therapy of low grade refractory/relapsed non-Hodgkin’s lymphoma,” SNM’s 56th Annual Meeting, June 13–17, 2009.
For more information: www.snm.org


 February 13, 2026
February 13, 2026 









