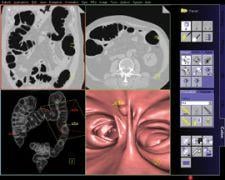
The syngo Colonography PEV (Polyp Enhanced Viewing) by Siemens Medical Solutions is an automated second reader tool for the visualization of lesions in the colon. The solution helps radiologists to detect polyp-shaped objects between 6 millimeters and 25 millimeters in size and can now be used both in clean-prepped and solid-liquid tagged protocols.
Siemens will feature the syngo MammoCAD that was created for full-field digital mammography and the detection of masses and microcalcifications to help radiologists detect breast cancer in its earliest and most treatable form. The system reportedly provides low rates of false positive marks with up to 40 percent of normal cases having no false positive detections at all.
The syngo Lung CAD software was created for automated detection of solid lung nodules in thoracic CT studies and reportedly has improved detection performance for solid nodules (the next generation version is works-in-progress and not yet available for clinical use). syngo Lung CAD is based on proprietary image processing and pattern recognition algorithms that have reportedly been extensively trained on a large database of thoracic CT studies.


 June 06, 2023
June 06, 2023 









