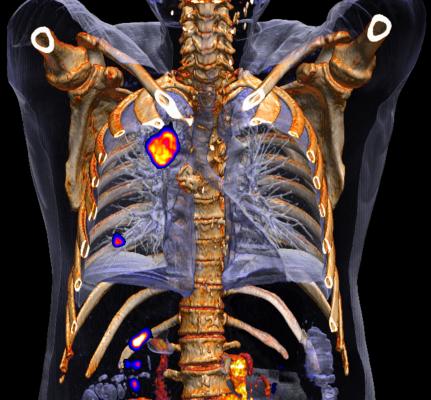
June 12, 2015 - Siemens' PETNET Solutions Inc. has entered into a nationwide agreement with U.K.-based Blue Earth Diagnostics Ltd. for the exclusive commercial manufacturing and distribution of Fluciclovine (18F), an investigational positron emission tomography and computed tomography (PET/CT) radiopharmaceutical. Fluciclovine (18F) is being studied for prostate imaging in clinical trials conducted in the United States, Japan, Italy, Norway, Sweden and Finland. The F-18-based radiopharmaceutical has a long half-life, which may facilitate its geographical distribution to clinical trial sites and then to clinical imaging centers once it gains approval from the U.S. Food and Drug Administration (FDA).
Prostate cancer is the second-leading cause of cancer in men worldwide, with 220,800 men expected to be diagnosed in 2015 in the U.S. alone. Currently, approved diagnostic options for prostate cancer include PET/CT scans using an injectable radiopharmaceutical to evaluate the extent of metastatic disease specifically for soft tissues and/or bony anatomy.
With its large commercial radiopharmacy network in the U.S., Siemens' PETNET Solutions provides wide access to PET radiopharmaceuticals for oncology, cardiology and neurology to imaging physicians and patients. Blue Earth Diagnostics Ltd. is a private U.K.-based diagnostic company backed by Syncona Partners LLP, a subsidiary of the Wellcome Trust that is developing and commercializing PET agents for cancer.
For more information: www.siemens.com


 February 13, 2026
February 13, 2026 









