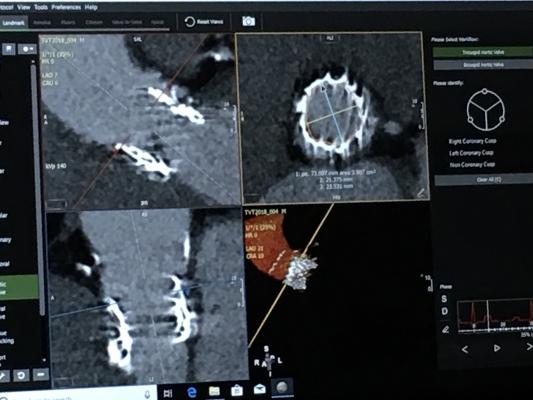
January 8, 2019 — The Society of Cardiovascular Computed Tomography (SCCT) has released a new expert consensus document for computed tomography (CT) imaging to improve outcomes for patients undergoing transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). The 2019 guideline, a result of a consensus statement of experts in the field, was published in the Journal of Cardiovascular Computed Tomography.1 The new recommendations are an update to the 2012 SCCT consensus document on computed tomography imaging in context of TAVI/TAVR.
“The 2019 consensus statement represents a contemporary assessment of the role of computed tomography imaging that will serve as important guidance to a field that has seen rapid growth over the last six years,” said consensus statement author Jonathon A. Leipsic, M.D., FSCCT, of the University of British Columbia. “This guidance will in turn help ensure the appropriate use of CT to help inform clinical practice and continue to drive improvements in clinical outcomes for the patients we serve.”
TAVI/TAVR is a treatment strategy for patients with symptomatic severe aortic stenosis (narrowing of the exit of the left ventricle). The option for the non-surgical TAVI/TAVR treatment has now expanded from those who are ineligible for surgery or high-risk surgical candidates to also include patients who are at an intermediate risk for conventional surgical valve replacement.
“The role of computed tomography, which was initially used primarily for evaluating peripheral access, has grown substantially,” said author Philipp Blanke, M.D., FSCCT, of the University of British Columbia. “It is now the gold standard for accurately sizing the annulus, determining the risk of annular injury and coronary occlusion and providing predictions in advance of the TAVI/TAVR procedure.”
The updated consensus statement has been developed to better reflect the substantial volume of new data that has been published since 2012 describing the use of computed tomographic angiography in TAVI/TAVR planning and post-procedural assessment. The 2019 consensus document includes:
-
Recommendations for CT acquisition prior to TAVI/TAVR;
-
Recommendations for the sizing and reporting of the aortic valve, annulus and outflow tract;
-
Recommendations for the reporting of fluoroscopic angulation;
-
Recommendations for the reporting of vascular access, coronary artery, and non-cardiac, non-vascular findings; and
-
Recommendations for the reporting of post- TAVI/TAVR and pre-VIV scans
The recommendations were written by 11 experts and based on the level of consensus: strong (≥9 in agreement), moderate (7-8) and weak (6). Recommendations with less than six of the author group in support of the statement were not adopted into the 2019 expert consensus document.
For more information: www.scct.org
Reference
1. Blanke P., Weir-McCall J.R., Achenbach S., et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI) / transcatheter aortic valve replacement (TAVR): An expert consensus document of the Society of Cardiovascular Computed Tomography. Journal of Cardiovascular Computed Tomography, Jan. 7, 2019. DOI: https://doi.org/10.1016/j.jcct.2018.11.008


 February 13, 2026
February 13, 2026 









