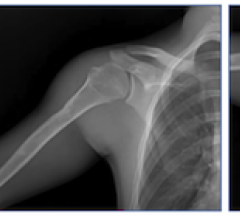
February 8, 2018 — Planmed announced the launch of the improved Planmed Verity cone beam computed tomography (CBCT) scanner for orthopedic as well as head and neck imaging. The scanner now offers enhanced image quality, new options for head and neck imaging, Planmeca Ultra Low Dose imaging protocol for lower patient doses, and Planmeca CALM algorithm for motion artifact correction.
Image quality of the mobile CBCT system has been enhanced to meet all imaging needs, according to Planmed. The high-quality images of Planmed Verity visualize even the smallest bone structures with minimal interference. This image quality combined with new patient and volume positioning options makes it possible to acquire images of the head and neck region with greater precision than before. Planmed Verity covers ear, nose and throat imaging as well as basic 3-D dental imaging needs.
The Planmeca CALM motion artifact correction algorithm eliminates the need for retakes by cancelling the effects of patient movement, which makes it ideal for imaging more lively patients. This feature will not only save time for clinicians but also guard patients from unnecessary doses, according to the company.
Also available now is the Planmeca Ultra Low Dose (ULD) imaging protocol. Planmeca ULD combines clinical image quality with extremely low effective patient doses, enabling 3-D imaging with a dose close to standard 2-D imaging. The protocol allows reducing the Planmed Verity scanner’s already low effective patient doses by 50 percent without compromising diagnostic image quality. Also, the new volume positioning possibilities enable enhanced accuracy to the imaging area, reducing the amount of radiation patients are exposed to.
The new features are now available inside the European Union and other countries in the European Economic Area where CE mark applies.
For more information: www.planmed.com


 March 03, 2026
March 03, 2026