April 16, 2012 — Piramal Healthcare Ltd. has signed an agreement to acquire worldwide rights to the molecular imaging research and development portfolio of Bayer Pharma AG through its newly created subsidiary, Piramal Imaging SA. The portfolio includes rights to florbetaben, which is in the final stages of its Phase III clinical trials. First Phase III results will be presented April 25, 2012, at the American Academy of Neurology's 64th Annual Meeting in New Orleans.
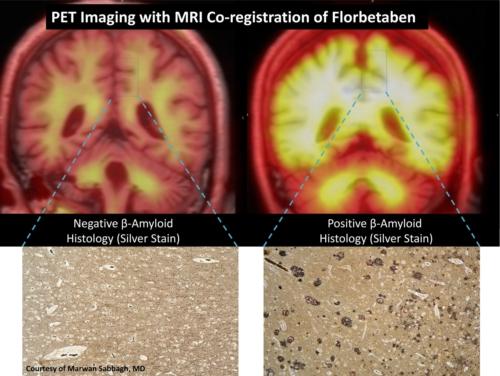
Florbetaben is a positron emission tomography (PET) tracer for the detection of beta-amyloid plaque deposition in the brain, which is the pathological hallmark of disease in probable Alzheimer's disease (AD) patients. Detection of beta-amyloid depositions is expected to result in earlier diagnosis and more specific treatment of AD.
The Phase III trial showed that PET imaging with florbetaben reliably detects beta-amyloid in the brain during life with great accuracy, thus showing value as a potential tool to aid in the diagnosis and assessment of AD. All study endpoints were met. The visual assessment procedure proposed for routine clinical practice demonstrated 100 percent sensitivity and 92 percent specificity with excellent inter-reader agreement (kappa = 0.88).
"This is the second acquisition of late stage assets after our acquisition of assets of BioSyntech in 2011, where we have recently received the European CE mark approval for an innovative bio-orthopedic product for cartilage repair, BST-CarGel, which enables the company to commercialize BST-CarGel in all of the countries in the European Union. We plan to build a promising portfolio in the pharma space, including our newly acquired molecular imaging assets, which will help us create a global branded pharma business," said Ajay Piramal, chairman of Piramal Group.
As per the agreement, Piramal will have the intellectual property (including patents, trademarks and know-how), worldwide development, marketing and distribution rights of the lead compound florbetaben as well as other clinical and pre-clinical assets of Bayer's molecular imaging business.
Piramal estimates that the new class of PET imaging agents for AD has a global market potential of up to $1.5 billion and is setting up a dedicated global commercial team for florbetaben. Core members of Bayer's research and development team working on the portfolio will be joining Piramal Imaging, which will carry forward the development of florbetaben and take it through regulatory approval processes worldwide. Piramal is planning to file for regulatory approvals in 2012.
Historically, the only way to definitively diagnose AD has been after death at autopsy, through analysis and identification of beta-amyloid in brain tissue. The Phase III trial employed a unique and rigorous study design comparing in vivo brain PET imaging with florbetaben to postmortem analysis of brain tissue.
"This is not only an important milestone for florbetaben, but it also marks an important milestone for our company," said Swati A. Piramal, director, Piramal Healthcare Ltd. "The creation of Piramal Imaging allows us to pursue our mission to increase diagnostic accuracy of serious medical conditions for improved patient outcomes."
The trial is the first to overlay MRI (magnetic resonance imaging) and PET data to accurately match florbetaben gray matter uptake with disease in six defined regions of the brain. This was done to confirm that florbetaben binds to beta-amyloid on both a regional (brain sections) and subject (whole brain) level. This combination provides considerably more data points than any other beta-amyloid tracer trial to date. Based on a region-by-region comparison, florbetaben detected beta-amyloid with a sensitivity of 77 percent and a specificity of 94 percent with substantial inter-reader agreement (kappa = 0.66), significantly exceeding the pre-specified threshold, confirming the study hypotheses.
For more information: www.piramalhealthcare.com


 February 13, 2026
February 13, 2026 









