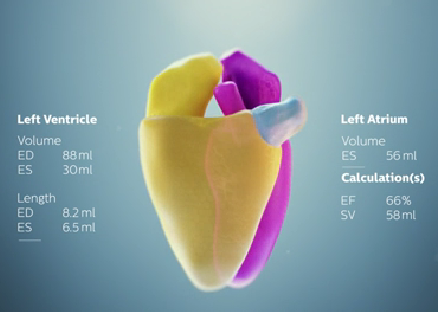
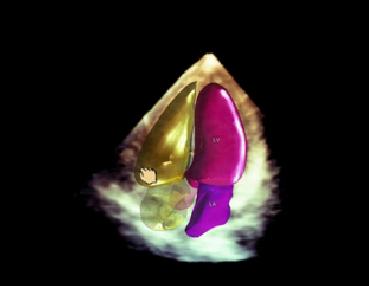
June 16, 2015 - Royal Philips announced the introduction of HeartModelA.I., a new Anatomically Intelligent Ultrasound (AIUS) tool that brings advanced quantification, automated 3-D views and robust reproducibility to cardiac ultrasound imaging. Philips' fastest 3-D AIUS ultrasound measurement method was unveiled on its Epiq 7 ultrasound system during the American Society of Echocardiography (ASE) annual meeting in Boston.
Using HeartModelA.I., clinicians can quickly, easily and confidently assess disease states, determine treatment and guide related therapies. In a recent comparison, exams with HeartModelA.I. were shown to be three to six times faster than conventional 2-D exams in gathering left ventricular and atrial dimensions and volumes (LV and LA), while offering the many benefits of 3-D imaging. With a rich digital database of anatomical structural models and adaptive system technology, HeartModelA.I. has access to advanced clinical information that automatically adapts to variability in patient anatomy. This knowledge-base identification and patient-specific adaptation provides proven quantification of the left ventricle and atrium, and display of routine apical views.
HeartModelA.I. is part of a suite of new tools and technologies available on Philips' Epiq 7 ultrasound system. The Epiq 7 system is designed to enhance automation and reproducibility to help address some of the most critical strains on overburdened hospitals and healthcare systems challenged to provide higher quality care at a lower cost. Epiq 7 is known for its high-image quality, advanced automation and reproducibility, and ergonomic design.
"Traditionally, collecting and analyzing heart measurements have been time-consuming, difficult processes with resulting variability that can impact diagnostic confidence," said Roberto Lang, M.D., professor of medicine and director of noninvasive cardiac imaging laboratories, University of Chicago Medicine. "Today's busy, constrained clinical environments need simplified methods to help provide high-quality care at low costs. Philips' HeartModelA.I. helps take the variability out of critical cardiac ultrasound measurements and enables time savings, broader applicability and accurate data to inform better delivery of care."
HeartModelA.I. will be made available on the Epiq 7 ultrasound system in the United States beginning in August and globally by the end of the year.
For more information: www.philips.com


 March 06, 2026
March 06, 2026 









