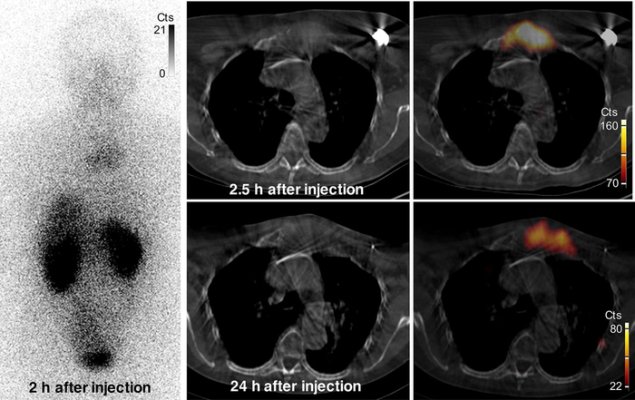
Images of uptake in bone metastasis in patient 2. Anterior whole-body (left) and SPECT/CT (right) images show uptake of 131I-GMIB-anti-HER2-VHH1 at level of large lytic bone metastasis, with soft-tissue component at sternal bone both at 2.5 h and at 24 h after injection. Small differences in area of uptake are explained by differences in patient positioning (arms up at 2.5 h and arms down at 24 h, to maximize patient comfort). Cts = counts. Image created by M. Keyaerts, University Hospital of Brussels (UZ Brussel), Belgium.
October 5, 2021 — A promising radionuclide treatment may offer new therapeutic options for breast cancer patients, according to research published in The Journal of Nuclear Medicine. The treatment combines radioactive iodine therapy with single-domain antibodies that target the human epidermal growth factor receptor type 2 (HER2) antigen to destroy cancer cells. This first-in-human study demonstrates the safety and efficacy of the radionuclide in patients with HER2-positive breast cancer.
Treatment of metastatic HER2-positive breast cancer has made great progress in recent years; nevertheless, the disease remains incurable. Today, no HER2-targeting radiopharmaceuticals are available to patients. With the development of the new targeted radionuclide theranostic agent, 131I-GMIB-Anti-HER2-VHH1, researchers sought to evaluate its safety, biodistribution, radiation dosimetry and tumor-imaging potential with the goal to introduce a new mechanism of action in the treatment of HER2-positive breast cancer.
In the study, six healthy volunteers and three HER2-positive breast cancer patients received injections of 131I-GMIB-Anti-HER2-VHH1. Vital signs and bloodwork, as well as open-ended questions, were used to assess the safety of the radionuclide. Whole-body imaging was conducted to ascertain biodistribution information and the resulting image data determined the dosimetry. In the three subjects with breast cancer, imaging was also used to measure tumor uptake.
131I-GMIB-anti-HER2-VHH1 was found to be safe and stable after administration and to clear rapidly from the blood in healthy volunteers. The tracer accumulated in metastatic sites of patients with HER2-positive breast cancer.
“This research is the first step in the clinical development of 131I-GMIB-Anti-HER2-VHH1 for the treatment of this devastating cancer indication, said Marleen Keyaerts, M.D., Ph.D., nuclear medicine physician at the University Hospital of Brussels in Jette, Belgium, and principal investigator of the phase I study. “As a result of this study, multicenter dose escalation and therapeutic clinical investigation of 131I-GMIB-anti-HER2-VHH1 in patients with HER2-positive breast and gastric cancer is underway.”
According to study sponsor Tony Lahoutte, M.D., Ph.D., chief scientific officer of Precirix, single-domain antibodies, such as 131I-GMIB-Anti-HER2-VHH1, could prove to be very beneficial in the future. “Single-domain antibodies are attractive probes for targeted radionuclide therapy and imaging due to their high binding specificity, sub-nanomolar affinity and low immunogenicity. The findings outlined in this publication demonstrate the potential of iodinated single domain antibodies to serve as imaging agent and therapy for cancer patients,” said Lahoutte.
For more information: www.snmmi.org


 March 06, 2026
March 06, 2026 









