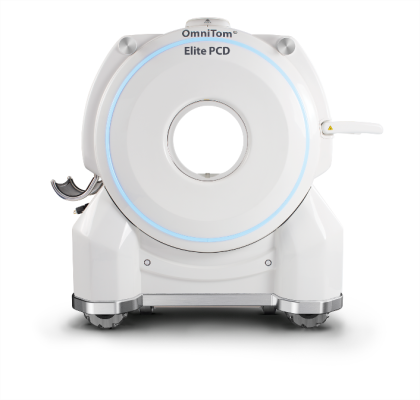
March 11, 2022 — The world of medical imaging is no longer just black and white. NeuroLogica Corp., a subsidiary of Samsung Electronics Co. Ltd., announced today its state-of-the-art OmniTom Elite has received 510(k) clearance for the addition of Photon Counting Detector (PCD) technology. NeuroLogica delivers the first FDA 510(k) cleared, single-source photon counting computed tomography (CT) scanner with single detector on a mobile system. OmniTom Elite with PCD can generate spectral CT images at multiple energy levels.
Photon counting is a next-generation CT technology that sorts the different energies of X-rays after they have passed through the scan field. A single X-ray source paired with PCD generates multiple sets of CT data acquired at the same time with configurable energy thresholds without any cross talk between images. PCD provides the ability to capture CT data in multiple energy bands leading to potentially more accurate visualization and segmentation of bone, blood clots, plaque, hemorrhage, and intracranial tumors. There is also potential with PCD to lower the dose requirements, and fundamentally change the use of injected contrast.
“NeuroLogica is driven by innovation. Since the advent of the world’s first multi-slice mobile CT in 2004, we have always known that point-of-care imaging can improve patient outcomes and increase the likelihood of a better quality of life after a traumatic event,” said David Webster, Chief Operating Officer of NeuroLogica. “With the introduction of PCD technology to the OmniTom Elite platform, we look to expand the diagnostic possibilities of CT at the patient’s bedside.”
The OmniTom Elite has the ability to provide versatile, real-time mobile imaging to administer point-of-care CT to critical patients without the need to transport them to a separate imaging department. The mobile unit will decrease the time it takes to diagnose and initiate treatment for these critical patients.
“Availability of photon-counting detector technology on a mobile head CT platform is a significant advance in the development of CT,” said Rajiv Gupta, PhD, MD, Neuroradiology Division Chief of Massachusetts General Hospital and Associate Professor at Harvard Medical School. “This advance heralds a new era of CT applications in stroke, trauma, ICU and intra-op settings.” MGH is collaborating with NeuroLogica and will pilot test the OmniTom Elite with PCD to monitor post-trauma and post-surgical patients.
Upgrading capabilities were top of mind when designing OmniTom Elite mobile CT scanners. In the near term, all current OmniTom users will have the opportunity to upgrade their scanners with this technology. Meanwhile, a limited number of premier research partners will aid in developing the full potential of OmniTom Elite with PCD.
NeuroLogica’s OmniTom Elite, and the entire mobile CT product line are manufactured in Danvers, MA, USA.
For more information: www.neurologica.com
Related Photon-counting CT Content:
Mayo Clinic Begins Use of Third-Generation Photon-counting CT Clinical Research Detector
VIDEO: New Advances in CT Imaging Technology — Interview with Cynthia McCollough, Ph.D.
VIDEO: Photon Counting Detectors Will be the Next Major Advance in Computed Tomography — Interview with Todd Villines, M.D.
Key Trends in Cardiac CT at SCCT 2020
GE Healthcare Pioneers Photon Counting CT with Prismatic Sensors Acquisition
Top Trend Takeaways in Radiology From RSNA 2020
VIDEO: Advances in Cardiac CT Imaging — Interview with David Bluemke, M.D.


 March 09, 2026
March 09, 2026 









