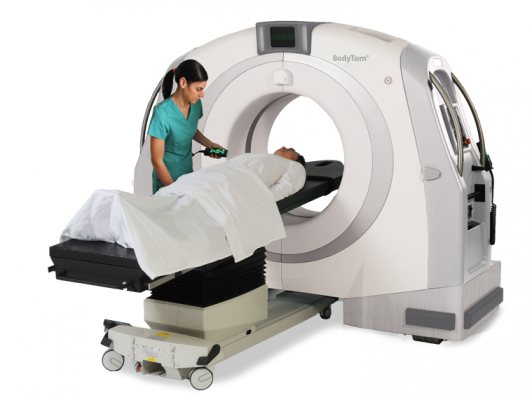
February 25, 2020 — With initial order of 10 mobile BodyTom computed tomography (CT) scanners ordered in China, Samsung NeuroLogica announced that it will dedicate significant resources to expediting manufacture and shipping of its scanners to help mitigate the growing coronavirus crisis in the country.
CT imaging showing evidence of viral pneumonia is recognized as the most effective means of diagnosing patients suspected of infection with the virus, without the false negatives of DNA testing, which have recently emerged.[1] Especially suited for screening a contagious disease, the 32-slice BodyTom’s portable, internally shielded design enables scanning of quarantined patients at the point-of-care, minimizing exposure of unaffected individuals to the disease during the exam process. The full-body, high image quality CT is compact and easily maneuverable, accommodating small spaces in cruise ships and other unusual scanning settings where patients are being kept in quarantine.
“As a mobile CT scanner with dose appropriate Lung Screening Protocols, BodyTom is the most effective solution for use in environments like they are faced with in China. Our Boston-based employees are working tirelessly to build as many scanners as possible to ship to the affected areas to help definitively diagnose patients,” said David Webster, COO for Samsung NeuroLogica. “In a recent study published in Radiology, CT helped to diagnose a number of cases that DNA testing did not pick up.[1] In one instance, even a second DNA test several days after the initial evaluation remained negative while signs of the coronavirus were immediately seen on CT.”
Study authors note that this, along with the slow turnaround time and limited DNA testing supplies in China, highlights the need for better screening for early stage disease. CT is particularly appropriate for patients with a high likelihood of virus infection.
With assistance from its Chinese distributor Chindex, NeuroLogica will mount a humanitarian effort to step up delivery of the BodyTom units, potentially from months to weeks following initial order throughout China to help meet that need.
BodyTom is a mobile, full-body, 32-slice CT with full diagnostic image quality. The system has an 85 cm gantry and 60 cm field of view and can accommodate patients of all sizes. The combination of rapid scan time, flexible settings, and immediate image viewing makes the BodyTom a valuable tool to all facilities needing versatile real-time mobile imaging.
[1] Xingzhi Xie, Zheng Zhong ,Wei Zhao, Chao Zheng, Fei Wang, & Jun Liu (2020). Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. Retrieved from https://pubs.rsna.org/doi/10.1148/radiol.2020200343
For more information: www.neurologica.com
Additional References on the Novel Coronavirus:
1. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing: Radiology. https://doi.org/10.1148/radiol.2020200343. Accessed Feb. 14, 2020
2. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia: Radiology. https://doi.org/10.1148/radiol.2020200370. Accessed Feb. 14, 2020
3. U.S. Food and Drug Administration: https://www.fda.gov/news-events/press-announcements/fda-takes-significant-step-coronavirus-response-efforts-issues-emergency-use-authorization-first?utm_campaign=020420_PR_FDA%20Issues%20EUA%20for%20First%202019%20Novel%20Coronavirus%20Diagnostic&utm_medium=email&utm_source=Eloqua. Accessed Feb. 14, 2020.
4. Public Health News Alert: CMS Develops New Code for Coronavirus Lab Test. https://www.cms.gov/. Accessed Feb. 14, 2020.
Related Coronavirus Imaging Content:
Radiologists Describe Coronavirus CT Imaging Features
CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia
Infervision in the Frontlines Against the Coronavirus
CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV)
Chest CT Findings of Patients Infected With Novel Coronavirus 2019-nCoV Pneumonia


 February 16, 2026
February 16, 2026 









