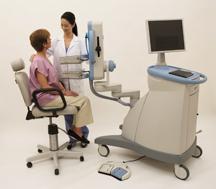
June 5, 2007 - Naviscan took the stage at SNM to showcase the PEM Flex Solo II, an organ specific, high resolution PET scanner for breast cancer detection using positron emission mammography (PEM).
The metabolic imaging modality acquires 3-D tomographic images, and reportedly has proven ability to image lesions as small as 2 mm for early detection. Sensitivity and specificity are said to be greater than 90 percent. ( The Breast J, 2006: 12/4: 309-323) The system’s current applications and reimbursement are for surgical planning, neo-adjuvant treatment follow up, recurrence and as an adjunct to other imaging modalities for case clarification.
The system is currently involved in a prospective, multi-center, randomized, controlled clinical trial comparing the accuracy of PEM and MR in lesion detection and pre-surgical planning at Scripps Clinic, Johns Hopkins, Anne Arundel, University of North Carolina.


 February 13, 2026
February 13, 2026 









