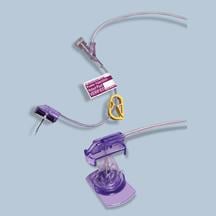
Xcela Power Injectable Port catheter for long-term delivery of fluids and medications including chemotherapy.

Xcela Power Injectable Port catheter for long-term delivery of fluids and medications including chemotherapy.
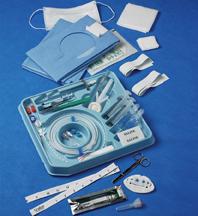
Xcela Power Injectable Port catheter for long-term delivery of fluids and medications including chemotherapy.
March 11, 2009 - Navilyst Medical is highlighting recently released products that included PICC Convenience Kitting, Xcela Power Injectable Port catheter for long-term delivery of fluids and medications including chemotherapy, and the EZ Huber Safety Infusion Set, which offers a dual-action safety mechanism designed to reduce the risk of bloodborne pathogen exposure.
The products provide clinicians and hospitals the catheter and accessory choices they require for a procedure that is efficient and conducive to reducing the risk of accidental needlesticks (OSHA) and infections (CDC and Joint Commission). This also reduces costs from unnecessary supplies, the company said.
The company expanded its customized PICC Convenience Kit to include the new Xcela Power Injectable PICC and a wider array of PICC insertion accessories and packaging. The titanium Xcela ports, available in standard and low profile sizes, offer durability and reduced size for ease of implantation and patient comfort, the company claims. The plastic Xcela ports are lightweight for patient comfort and offer radiolucence-reduced imaging artifact. The plastic/titanium hybrid port design option combines the durability of titanium with the lightweight and radiolucence of plastic. All designs have a 5mL/sec with 300 psi infusion rating for use in contrast imaging procedures
Navilyst Medical’s new EZ Huber Safety Infusion Set offers a dual-action safety mechanism designed to reduce the risk of bloodborne pathogen exposure that threatens clinician safety and is a significant cost to the healthcare system. One safety feature shields the tip of the needle after the needle is withdrawn from the patient to prevent accidental needle sticks. The second safety feature is a protective cover that surrounds the entire needle after withdrawal to reduce exposure to aerosolized or splattered infusion fluids and blood.
The EZ Huber Safety Infusion Set is compatible with Navilyst Medical’s new Xcela Power Injectable Port and is rated for up to 5mL/sec at 300 psi for contrast-enhanced CT imaging procedures.
For more information: www.navilystmedical.com



 January 13, 2026
January 13, 2026 









