May 3, 2021 — Molli Surgical Inc. announced U.S. Food and Drug Administration (FDA) clearance for Molli, a new wire-free localization technology for breast cancer surgery. The new technology helps radiologists mark lesions quickly and accurately, and enables surgeons to locate and remove lesions more efficiently while facilitating the best cosmetic results and a better patient experience.
“This year, alone, more than two million women around the globe will receive the devastating news that they have breast cancer,” said Fazila Seker, Ph.D., CEO of Molli Surgical. “Our mission is to make their journey smoother and support them along the way. Our new Molli technology will make breast cancer surgery easier and more precise as it helps healthcare providers locate and remove even the smallest tumors with unprecedented precision.”
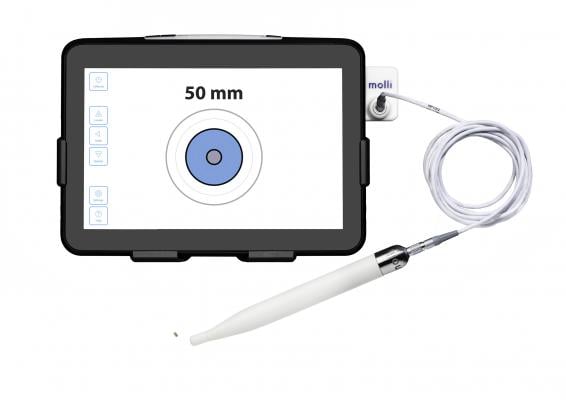
Molli consists of an implantable marker, detection wand and visualization tablet that together, provide superior tumor localization. The technology, which is used by both radiologists and surgeons, is reliable, easy-to-use, and provides more efficient clinical workflows compared to wire or radioactive seed methods. Molli uses non-radioactive, solid magnet technology to mark target tissue on the same day or less than 30 days prior to surgery. During the procedure, the surgeon uses the Molli Wand, which detects the Molli Marker, to remove the lesion more efficiently.
“Unlike radioactive seed localization, Molli eliminates any anxiety patients may have related to radiation exposure from radioactive seed localization methods,” said Ananth Ravi, Chief Science & Clinical Officer, Molli Surgical. “Because Molli is based on magnetism, not radiation, hospitals avoid the time-consuming and expensive resources to adhere to radiation safety regulations. With Molli, there are no complex monitoring and disposal protocols, cumbersome and expensive equipment or lost time searching for a misplaced radioactive seed.”
“Molli began at the Louise Temerty Breast Cancer Centre at Sunnybrook Hospital when a patient advisory council challenged Ravi’s group to come up with a kinder, more accurate and cost-effective alternative to wire-guided localization,” said Andy Smith, President and CEO of Sunnybrook Hospital. “I want to congratulate the entire Molli Surgical team for this remarkable achievement in surgical innovation — but, more importantly, for the enormous and worldwide impact they are making for people living with breast cancer.”
Molli provides better accuracy with wire-free localization, without the radiation of other methods and the technology has tremendous market opportunity with applications that extend beyond breast cancer tumor localization. It’s easy-to-use and designed to work with standard clinical workflows and surgical instruments. The technology provides confidence and efficiency in radiology by utilizing the benefits of easy introducer needle insertion and standard marker deployment and is compatible with conventional mammography and ultrasound imaging. The Molli Marker is only 3.2 mm in size, allowing radiologists to accurately localize the smallest non-palpable tumors. The device’s solid magnetic technology means it can’t be deactivated and there is no radioactivity tracking or signal decay.
For more information: www.mollisurgical.com


 March 06, 2026
March 06, 2026 









