May 1, 2024 — Hologic, Inc., a global leader in women’s health, today announced that it signed a definitive agreement to acquire Endomagnetics Ltd (Endomag), a privately held developer of breast cancer surgery technologies, for approximately $310 million, subject to working capital and other customary closing adjustments.
“Endomag’s suite of solutions complements our existing breast surgery portfolio and will provide surgeons and radiologists with an expanded range of options to meet the individual needs of more patients undergoing critical breast cancer procedures,” said Erik Anderson, President of Breast and Skeletal Health Solutions at Hologic. “With a global footprint and a similar commitment to women’s health, we are excited about the potential of welcoming the Endomag team and our future opportunities together to increase access to these technologies and better serve patients across the breast health continuum of care.”
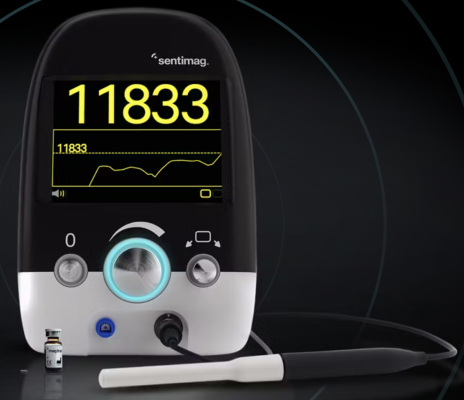
Endomag, which is based in Cambridge, United Kingdom, develops and sells breast surgery localization and lymphatic tracing technologies. Its products include the Magseed marker for magnetic tissue localization before surgery, the Magtrace lymphatic tracing injectable for breast cancer staging and the Sentimag platform, which supports both localization and lymphatic tracing.
“We are delighted to attract the strength and focus of Hologic to power the next phase of our growth,” said Eric Mayes, CEO of Endomag. “Our team is encouraged by our cultural alignment and the potential to strengthen a broader portfolio of solutions to improve women’s health globally.”
Endomag generated approximately $35 million of revenue in calendar 2023. The acquisition is expected to be slightly dilutive to Hologic’s non-GAAP earnings per share in fiscal 2024, break even in 2025 and accretive thereafter.
Completion of the acquisition is subject to customary closing conditions, including regulatory approvals.
For more information: www.hologic.com


 February 23, 2026
February 23, 2026 









