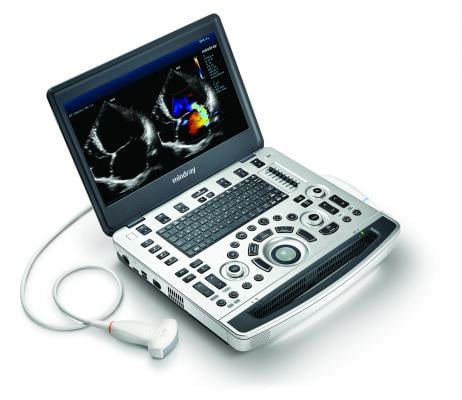
Oct. 29, 2014 — At the American College of Emergency Physicians (ACEP) annual meeting in Chicago, Mindray introduced the M9 premium compact ultrasound system, the next generation in point-of-care ultrasound technology. The M9 is designed to provide physicians imaging for immediate clinical management and decision-making.
The introduction of point-of-care ultrasound into the frontline of emergency and critical care medicine brings the service to the patient. It offers expanded flexibility, including broad general imaging and comprehensive cardiac applications appropriate for the fast-paced and demanding emergency department environment.
The M9’s single crystal transducer with 3T technology provides deeper penetration and sharper image quality, giving physicians confidence. This unique technology also supports the M9’s cardiac tissue tracking accuracy and effectiveness for real-time quantitative assessment of myocardial function. There’s a seven-second start-up from standby and more than three hours of battery life, all within a slim profile ergonomic cart.
M9’s other features:
· Echo Boost cardiac image optimization
· High Dynamic Range color flow Doppler
· Full suite of transducers including TEE
· 15.6" high-resolution 1920 x 1080 HD monitor
· Durable magnesium alloy case with spill resistant keyboard
· Standard built-in Wi-Fi
For more information: www.mindraynorthamerica.com


 February 05, 2026
February 05, 2026 









