Image courtesy of VivoSight
October 30, 2017 — Current skin cancer diagnosis can last a number of weeks and be very upsetting. However, a new imaging system developed by a group of European scientists could dramatically speed up the process and reduce the need for debilitating sentinel-lymph node biopsies by placing real-time cancer diagnosis in the hands of a dermatologist.
Multiple stages of skin cancer diagnosis can involve visiting a GP, being sent to a dermatologist for a skin biopsy, waiting for laboratory analysis, having a sentinel lymph node biopsy under general anaesthetic, and then having more tests if the cancer has spread.
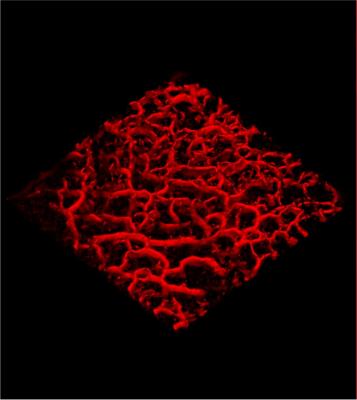
Now, using a handheld laser, a specialist can actually see under the skin at depths of 1 mm by creating a 3-D color image of the microscopic blood vessels in a process that takes around 30 seconds.
Employing a new and advanced version of optical coherence tomography (OCT), a photonics technique more commonly used in retina scans, the scanner captures 3-D images of the micro-structures under the skin with a harmless infrared laser beam.
Since melanomas need oxygen to grow and survive, they grow their own blood vessels. As the cancer develops and becomes more malignant, they become increasingly distorted and malformed, differing in appearance from healthy vessels.
Being able to detect and see these vessels in a suspicious lesion in real time has never been possible, until now, opening the possibility for dermatologists to make treatment decisions in an unrivalled timeframe.
Over 55,500 people in the world died from malignant melanoma of the skin in 2012 alone. While 2,459 deaths from melanoma skin cancer were recorded in the United Kingdom in 2014, an estimated 9,730 people will die of melanoma in the United States in 2017.
Melanomas produce their own blood vessels to feed and grow the tumor, so by revealing the microstructures in 3-D pictures the scanner show doctors how the cancer has developed.
A specialist can potentially determine on the spot, whether simply cutting it out is sufficient for a cure or whether further treatment with cancer drugs will be needed.
With their new device called VivoSight on the market, the European team ADVANCE (Automatic Detection of VAscular Networks for Cancer Evaluation) is aiming to reduce the time for treatment decision from a number of weeks to a matter of seconds, while removing the invasive nature of the appointment.
U.K. project leader Jon Holmes of Michelson Diagnostics Ltd, a key partner in the ADVANCE consortium, explained, “Every melanoma above a certain thickness could have spread to other parts of the body. At present, all patients with such melanomas have to wait for a sentinel lymph node biopsy performed in a hospital under general anesthesia to find out if it is spreading. This can take weeks to perform, is very expensive and can be debilitating for the patient.”
The ADVANCE team has employed a variant of OCT in its scanner called speckle-variance OCT or dynamic OCT (D-OCT), an advancement of OCT that is ideal for capturing movement.
Studying the ‘speckle’ or flicker of light patterns created by moving blood cells, the imaging device takes around four frames per second and compiles the images so that a clinician may tell where something has moved on the image from frame to frame.
“Using D-OCT we can see movement of blood against the solid tissue structures, something we have never been able to do before in a clinical setting. It’s like looking out at night and seeing cars’ headlights flowing along a motorway, only at depths of nanometers under the skin,” said Holmes.
While a sentinel node biopsy can cost in excess of €10,000 (~$13,000 U.S.), and with many hospitals performing hundreds per year, there is a growing concern and a need to find an alternative when over 80 percent of these operations turn out to be clear of any malignant growth, according to Holmes.
As well as its use in skin cancer diagnosis, the ability to see blood vessel networks with the ADVANCE technology has created a number of useful spin-off benefits.
“The scanner can image the blood vessels in healing wounds. This may have application for treatment of leg and foot chronic ulcers, when doctors want to know whether a wound is healing or requires a change in treatment, potentially reducing the number of amputations,” said Holmes. He added,
“ADVANCE technology may also help with burn victims, being able to give a doctor a quicker response time than the standard 15 days to determine whether a patient’s skin is healing and whether or not to give a skin graft.”
For more information: www.photonics21.org




 February 11, 2026
February 11, 2026