January 13, 2022 — Infinitt North America, an award-winning developer of enterprise imaging solutions for healthcare, today announced a partnership to market Lunit AI software, making it readily available to its North American customer base. Lunit recently received FDA 510 (k) clearance for Lunit Insight MMG, for detecting breast cancer, and Lunit CXR Triage, a Chest X-ray solution developed for sorting and prioritizing emergency cases.
By partnering with Lunit, Infinitt can offer an enhanced integration with the AI solutions, enabling radiologists and ER clinicians to apply the algorithms and machine learning seamlessly, without any disruption in workflow.
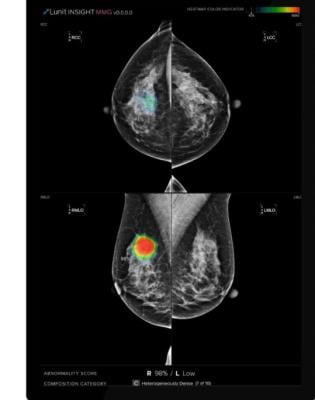
Developed using Lunit’s deep learning technology, Lunit Insight MMG accurately analyses mammography images to detect lesions that could indicate breast cancer and provides location information for any detected lesions in the form of outlines and heatmaps. The computer-assisted diagnostic software has shown excellent performance in the early detection of breast cancer.
Lunit Insight CXR Triage chest detection suite accurately detects pre-specified suspected critical findings in chest X-ray's (pleural effusion and/or pneumothorax), quickly identifying critical conditions needing immediate attention. The algorithm performs at a 98-99% accuracy rate.
David Smarro, President and CEO of Infinitt North America, is pleased to be partnering with a leading AI developer, marketing solutions that have already received FDA clearance.
“Artificial Intelligence has so much potential in imaging informatics, both as a diagnostic tool and in getting all relevant patient data to the right clinician or subspecialist as quickly as possible. Earlier and more accurate diagnosis, earlier treatment, and a more patient-centered approach to care will improve clinical outcomes and reduce healthcare costs,” said Smarro.
With Lunit Insight MMG, rapid triage of screening mammograms will filter out benign cases for later evaluation, helping to reduce strain on radiologists as 60% of screening mammograms come up normal.
For more information: www.infinittna.com/


 February 06, 2026
February 06, 2026 









