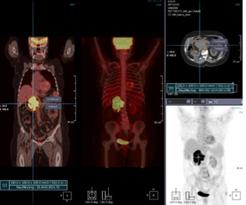
November 30, 2011 – Infinitt announced that its fusion software, Xelis Fusion, has received clearance from the U.S. Food and Drug Administration (FDA), and is now widely available to the North American market.
Xelis Fusion can be used as an integrated solution with Infinitt’s web-based picture archive and communications systems (PACS) or as a stand-alone solution, allowing any third-party PACS user to query, view and analyze studies from any digital imaging and communications in medicine (DICOM) database to do fusion of computed tomography (CT), positron emission tomography (PET) and magnetic resonance (MR) images.
According to the company, the Xelis Fusion product has been in use at 12 institutions across the United States and has demonstrated the ability to improve diagnosis and enhance the radiologist’s and clinician’s productivity. Edward Milman, M.D., director, MRI Services at St. Joseph's Regional Medical Center in Paterson, N.J., uses Xelis Fusion as a fully-integrated 3D application within the Infinitt PACS.
“I like Xelis Fusion because it integrates all the necessary tools to interpret a PET/CT,” Milman said. “I am able to access it directly from my PACS work list without third-party interference. There’s no need to interrupt the dictation. Key images are saved directly from the 3-D window into PACS and attached to the study to be accessed instantly.”
Xelis Fusion features:
· Supports fusion of PET, CT and MR data in any combination of the modalities
· Offers both automatic and manual registration methods, quantitative analysis tools and rapid image navigation using only a few mouse clicks
· Supports 3-D region of interest (ROI) analysis
· Provides a variety of presets and layouts for comparing two image sets
· Calculates PET Standard Uptake Values (SUV)
· Offers multiple standard and user-defined color maps
· Stores/sends the fused images in DICOM 3.0 format
· Includes integrated, freely-rotating MPR functions.
PET/CT Fusion is used primarily to assist in more accurate and reliable diagnosis of cancer, and is covered by most private insurance companies and by Medicare. Co-registration and alignment of different scans showing the same patient and pathology can add value to numerous image-based clinical review processes.
For more information: www.infinittna.com


 February 13, 2026
February 13, 2026 









