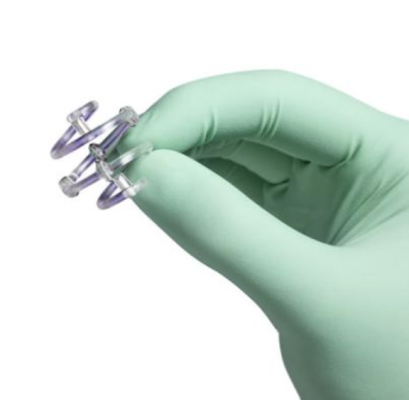
The U.S. Food and Drug Administration (FDA) has issued a recall of the Hologic, Inc. BioZorb marker due to complications with implanted devices. The agency identified this as a Class I recall, the most serious type of recall, in a May 22 written notice by the FDA shared here. Image courtesy: Hologic, Inc.
May 22, 2024 — The U.S. Food and Drug Administration (FDA) has issued a recall of the Hologic Inc. BioZorb marker due to complications with implanted devices. The agency identified this as a Class I recall, the most serious type of recall. Details issued in a written notice by the FDA on May 22 and are shared here.
The U.S. Food and Drug Administration (FDA) has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death. Details on the recall were issued in a written notice by the FDA and are shared here in their entirety.
Please be aware, this recall is a correction, not a product removal.
Recalled Product
Product Name: BioZorb Marker
Product Codes: NEU
Model Numbers
F0405 BioZorb Marker 4cm x 5cm
F0404 BioZorb Marker 4cm x 4cm
F0331 BioZorb Marker 1cm x 3cm x 3cm
F0231 BioZorb Marker 1cm x 3cm x 2cm
F0221 BioZorb Marker 1cm x 3cm x 2cm
F0304 BioZorb Marker 3cm x 4cm
F0303 BioZorb Marker 3cm x 3cm
F0203 BioZorb Marker 2cm x 3cm
F0202 BioZorb Marker 2cm x 2cm
Distribution Dates: April 29, 2019 to April 1, 2024
Devices Recalled in the U.S.: 53,492
Date Initiated by Firm: March 13, 2024
Device Use
The BioZorb Marker made by Hologic (previously Focal Therapeutics), is an implantable radiographic marker used to mark soft tissue (such as breast tissue) for future medical procedures, such as radiation. Device has two components: a permanent component which is made of titanium metal and a resorbable component which is made of a plastic material that resorbes over time. It's provided sterile, meant for one-time use.
Reason for Recall
Hologic, Inc. is recalling Biozorb Marker due to complications and adverse events reported with implanted devices. Complaints included reports of pain, infection, rash, device migration, device erosion, seroma, discomfort, or other complications from feeling the device in the breast, and the need for additional medical treatment to remove the device.
There have been 71 reported injuries and no reports of death.
Who May be Affected
- People who were implanted with the BioZorb marker device.
- People who receive radiation guided by the BioZorb marker which may have migrated.
- People who receive systemic cancer treatments as treatments may be delayed due to complications with BioZorb Marker.
- Radiologist, surgeons, oncologists and other health care providers who use BioZorb Marker for treatment of their patients.
What to Do
On March 13, 2024, Hologic, Inc. sent all affected customers an Important Medical Device Safety Notification.
The letter requested patients to:
- Contact their health care provider if they experience any adverse events following the placement of a BioZorb Marker.
- Discuss the benefits and possible risks of implantable breast tissue markers for breast cancer procedures with their health care provider.
- Report any problems or complications experienced following the placement of BioZorb Marker devices to Hologic at [email protected] and to the FDA’s MedWatch Adverse Event Reporting program.
- Discuss the benefits and possible risks of implantable breast tissue markers for breast cancer procedures with their health care provider.
The letter requested health care providers to:
- Be aware of reports of serious adverse events following the placement of the BioZorb Marker devices in breast tissue.
- Discuss the benefits and possible risks of BioZorb Marker devices with each patient.
- Inform all patients on which device will be used if a marking device will be used during breast conservation surgery.
- Continue to monitor patients who have an implanted BioZorb Marker for signs of any adverse events.
- Report any problems or complications experienced by patients following placement of the BioZorb Marker devices to Hologic
Complaints can be submitted to [email protected] and to the FDA’s MedWatch Adverse Event Reporting program.
Contact Information
Customers in the U.S. with questions about this recall should contact Hologic, Inc. at [email protected].
Additional Resources
Class 1 Device Recall BioZorb LP Marker (fda.gov)
BioZorb Markers and Potential Risks with Use in Breast Tissue: FDA Safety Communication | FDA
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.


 March 06, 2026
March 06, 2026 









