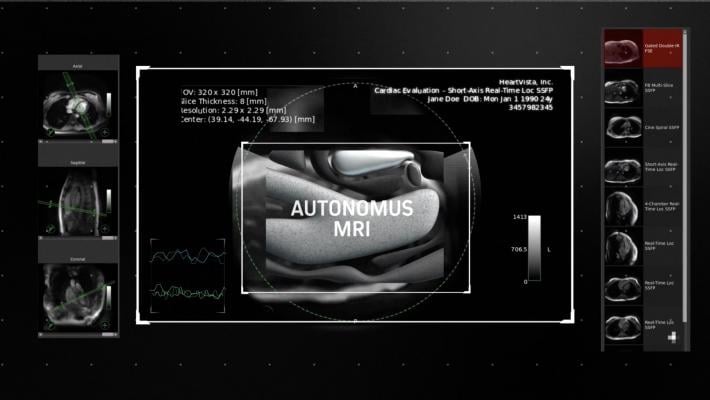
November 26, 2018 — HeartVista announced its artificial intelligence (AI)-driven, One-Click Autonomous MRI acquisition software for cardiac exams. Integrated with existing magnetic resonance imaging (MRI) scanners, the software uses AI to guide image acquisition.
With a single click, MRI technologists can perform a cardiac ischemia exam in less than 15 minutes using HeartVista's AI-driven software, compared to the traditional 90-minute session.
HeartVista's software automatically prescribes the standard cardiac views in as little as 10 seconds while the patient breathes freely. An artifact detection algorithm is incorporated in HeartVista's autonomous protocol to detect when the image quality is below the acceptable range, prompting the operator to reacquire the questioned images if desired. An accelerated non-Cartesian 4-D flow sequence is acquired in minutes, leaving enough time for the necessary calibrations prior to the myocardial delayed-enhancement acquisition. The smart in-line cardiac analysis package provides preliminary measurements of left ventricular function.
With HeartVista's AI-driven solution, barriers for wider adoption of cardiac MRI exams may be lowered. Patients may benefit from fewer breath holds and reduced discomfort, while increasing access to imaging for patients with arrhythmia, intolerance to long exams and other health constraints. Technologists benefit from the reduced complexity of exams, while clinicians can remotely monitor and guide the exams in real time and review scans acquired with consistency and confidence.
"We are excited to be one of the early adopters of HeartVista's groundbreaking autonomous MRI solution." said Graham Wright, Ph.D., director of the Schulich Heart Research Program at the Sunnybrook Health Sciences Center in Toronto. "The HeartVista Autonomous MRI solution will allow us to accelerate our design and development of streamlined cardiac MRI exams and innovative minimally invasive cardiovascular therapies."
HeartVista is introducing One Click Autonomous MRI at the Radiological Society of North America (RSNA) annual meeting, Nov. 25-30 in Chicago. HeartVista is also presenting with its partners Siemens and NVIDIA at RSNA 2018.
Device availability for certain features is pending U.S. Food and Drug Administration (FDA) 510(k) premarket clearance.
For more information: www.heartvista.com


 February 13, 2026
February 13, 2026 









