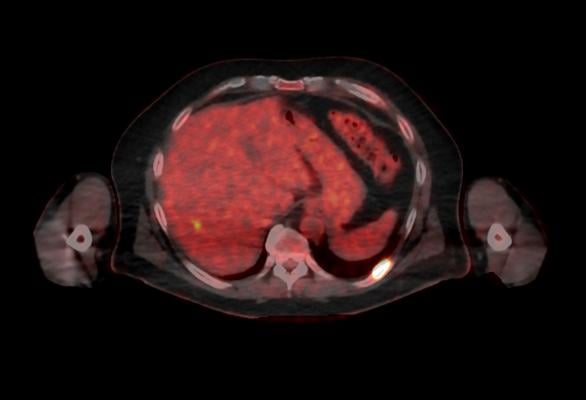
October 5, 2016 — GE Healthcare announced U.S. Food and Drug Administration (FDA) 510(k) clearance of its Discovery MI digital positron emission tomography (PET)/computed tomography (CT) system and shared a series of first clinical images. Built with technology allowing significantly better small lesion detectability, Discovery MI can help clinicians in their efforts to diagnose and stage disease earlier.
The first unit was installed at the Stanford University School of Medicine, Stanford, Calif.
Discovery MI was created to help clinicians achieve their primary goal of diagnosing and staging disease earlier and to better guide their treatment strategies. It also enables more compelling research with more novel, faster decaying tracers. A key component in delivering these desired outcomes is Discovery MI’s LightBurst Digital Detector. This detector delivers up to two times improvement in volumetric resolution, enabling small lesion detectability, and has the highest NEMA sensitivity of any time-of-flight (TOF)/PET system in the industry, according to GE.
Discovery MI may also enhance clinicians’ diagnostic service offerings in oncology and expand PET’s impact in neurology, cardiology and beyond. These expanded capabilities are enabled by the ability to increase low-yield tracer capability with protocols that reduce dose by up to 50 percent, allowing clinicians to pursue research without impacting image quality.
This system also features diagnostic CT innovations helping to deliver 100 percent better spatial resolution, with no increase in image noise with ASiR-V. And with Smart Metal Artifact Reduction (MAR), Discovery MI helps virtually eliminate streaks and shadows from metal artifacts, saving valuable time previously spent correcting images, increasing the number of successful scans for patients.
For more information: www.gehealthcare.com


 February 13, 2026
February 13, 2026 









