March 8, 2018 – Fujifilm Medical Systems U.S.A. Inc., a provider of diagnostic imaging products with a comprehensive portfolio of women’s health solutions, will unveil its latest mammography diagnostic workstation, the Aspire Bellus II, at the National Consortium of Breast Centers (NCoBC) conference, March 9 -14, 2018, in Las Vegas and the Society of Breast Imaging (SBI) Symposium, April 12 -15, 2018, also in Las Vegas.
The Aspire Bellus II smart mammography workstation offers a multimodality view for radiologists in a hi-resolution display, and also offers 2-D and tomosynthesis comparisons. While supporting a range of DICOM formats, the system is designed around the unique requirements of mammography. The Aspire Bellus II incorporates key diagnostic features such as current and prior image comparison, quadrant view, tomosynthesis support and invert. To optimize workflow the workstation enables customization for reading protocols and personalized configuration. It received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in September 2017 and will begin shipping to customers in spring 2018.
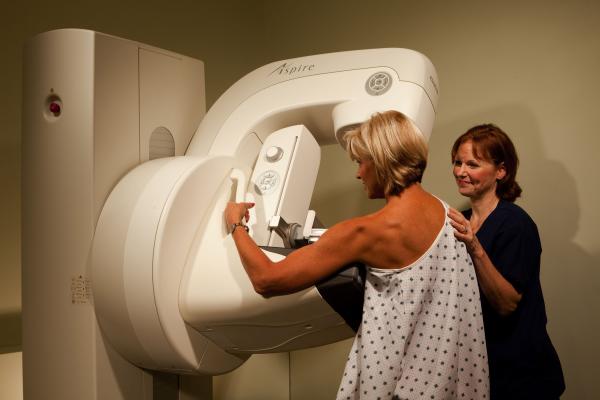
At both conferences, Fujifilm will also exhibit the Aspire Cristalle digital mammography system with digital breast tomosynthesis (DBT) option. It combines Hexagonal Close Pattern (HCP) image capture technology and intelligent image processing, optimizing both contrast and dose based on auto recognition of breast characteristics. This technology is designed to optimize image acquisition, regardless of breast composition and at a low dose. In addition, the Aspire Cristalle helps deliver noticeable patient comfort with its patented, flexible Comfort Paddle intended to provide gentle, even compression of the breast.
For more information: www.fujimed.com


 February 11, 2026
February 11, 2026 









