July 23, 2009 – The FDA recently cleared Ziehm Imaging’s latest mobile C-arm, the Ziehm Vision RFD, which is designed for use in endovascular surgery, interventional cardiology and interventional radiology.
The Ziehm Vision RFD mobile C-arm combines the latest flat-panel technology and a fully digital imaging chain with a compact design that delivers exceptional image quality, the company said.
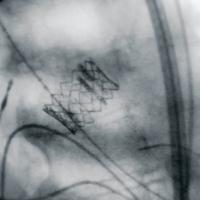
The new C-arm combines flat-panel technology with the sophisticated ODDC (object detected dose control) software and specially designed anatomical programs for good image quality at minimal dose. The flat-panel detector allows for fully digital, distortion-free imaging and better access to the patient due to the larger C-arm opening and the 165 degree orbital rotation. Thanks to pulse technology at up to 25 frames per second, the system provides sharp, high contrast images of vasculature, bone and soft tissue structures, which were once only available with fixed room systems, the company said.
The 30 x 30 cm size of the flat panel detector and the 1.5 x 1.5 k resolution provides extended dynamic range and 49 percent greater anatomical viewing, when compared to 12-inch image intensifier systems delivering comprehensive information about the patient’s anatomy. With the added advantage of a smaller footprint, users receive the benefit of greater maneuverability. At the same time, the mobile interventional suite provides better access to the patient due to the larger C-arm opening and the 165° orbital rotation. Workflow is simplified and day-to-day clinical efficiency is improved through the VisionCenter touchscreen user interface.
For more information: www.ziehm.com


 August 14, 2025
August 14, 2025