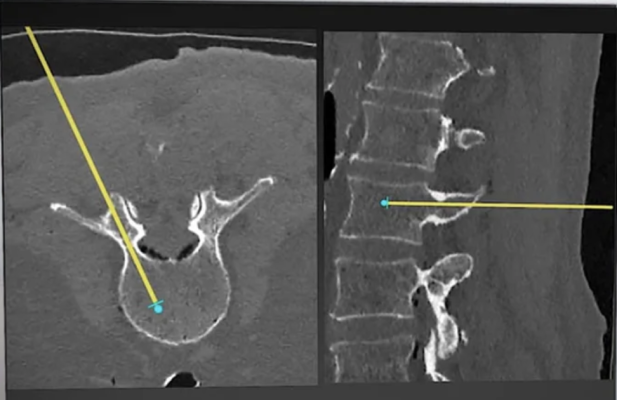
VUZE Medical has announced its receipt of U.S. Food and Drug Administration (FDA) 510(k) clearance for its second-generation VUZE System, which requires only a 2D C-arm and PC in the operating room, needing no cameras, markers, references or tool add-ons, reported the company. The privately-held Israel-based company provides software-based guidance and verification for minimally-invasive spine surgery. Image courtesy: VUZE Medical
May 30, 2024 — Vuze Medical, which develops medical technology to transform intra-operative guidance in spinal interventions currently aided only by X-ray, has announced its receipt of U.S. Food and Drug Administration (FDA) 510(k) clearance for its second-generation Vuze System. The privately-held Israel-based company provides software-based guidance and verification for minimally-invasive spine surgery.
With its newly-received FDA 510(k) clearance, medtech developer Vuze Medical reports that Vuze 2.0 provides much-extended interoperability and functionality, and, like Vuze 1.0, requires only a 2D C-arm and PC in the operating room, needing no cameras, markers, references or tool add-ons, reported the company.
The Vuze system’s current focus is on minimally invasive thoracolumbar stabilizations; however, noted the written statement issued on the clearance, the underlying Vuze technology is not specific to any particular anatomy, and the company intends to seek regulatory clearances for further spinal and skeletal interventions in the future. Vuze has already received eleven related patents in the U.S., Europe, China and India, including tie-ins with robotics, augmented reality (AR) and traditional hardware-based navigation.
The Vuze System is a software solution installed on an off-the-shelf PC. It operates with unmodified surgical tools, uses no markers, references or cameras, and makes 3D imaging in the operating room (OR) entirely optional. Using proprietary image processing algorithms, it overlays in real time graphical representations of standard surgical tools seen in intra-operative 2D X-ray images onto axial and sagittal cross-sections that it generates from the patient’s standard pre-operative 3D scan. The Vuze System received its initial U.S. FDA 510(k) clearance in 2022 and completed a first-in-human clinical trial in 2023.
The second-generation Vuze System supports a far broader range of surgical C-arms from all major vendors. Additionally, it accommodates more sources of 3D image data including both standard pre-operative CT and in-OR CT/CBCT scans. Its expanded functionality includes the ability to perform surgical planning at any time or place on a compatible standalone laptop, in addition to on the VUZE System itself.
“Our second generation significantly extends the applicability of the Vuze System across desired surgical workflows and operating room setups,” said David Tolkowsky, founder and CEO of Vuze Medical. He added, “In each of those situations, our aim is to preserve the advantages of common X-ray guidance while addressing its shortcomings.”
Over three million1 surgeries for correcting spinal instability and/or deformation, collectively known as spinal stabilizations, are performed annually worldwide, with as many as a third of those in the U.S.1 These procedures include vertebral fixation with pedicle screws, vertebral fixation coupled with fusion, and vertebral augmentation with synthetic or biological cement. The vast majority of stabilizations treat short spinal segments1. Short-segment surgeries are most often assisted only by standard 2D X-ray. The risk of pedicle screw misplacement has been shown to be greater under X-ray guidance than in navigated surgery 2,3.
Vuze Medical, based in Ra'anana, Israel, is a privately-held medical technology company that aims to provide highly accurate and cost-effective surgical guidance for common skeletal interventions currently aided only by standard 2D X-Ray, with a current focus on spine. The company’s Vuze System is a unique software-based solution that instantly merges intra-operative X-ray with pre-operative CT or CBCT, providing surgeons with the cross-sectional images they need most during surgery and traditionally lack. The system is targeted primarily at outpatient and ambulatory settings.
More information: www.vuzemedical.com
References:
1 Orthopedic Network News: 2020 & 2023 Spinal Surgery Updates
2 “Computer-Assisted Navigation Is Associated with Decreased Rates of Hardware-Related Revision After Instrumented Posterior Lumbar Fusion,” Bovonratwet et al., Global Spine Journal, May 2023
3. “Comparison of major spine navigation platforms based on key performance metrics: a meta-analysis of 16,040 screws,” Bonello et al., European Spine Journal, Sep 2023


 March 04, 2026
March 04, 2026 









