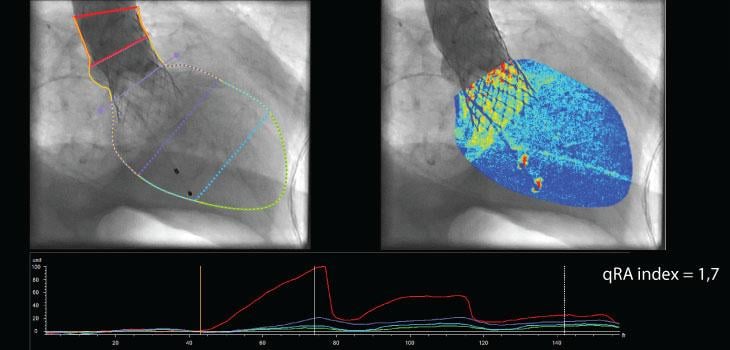
Image courtesy of Pie Medical Imaging
February 23, 2015 — Pie Medical Imaging BV announced that it received 510(k) clearance from the U.S. Food and Drug Administration for its CAAS A-Valve product including the quantitative Regurgitation Analysis (qRA) workflow. The qRA workflow is the first 510(k)-cleared image analysis technology to determine aortic regurgitation based on X-ray angiography.
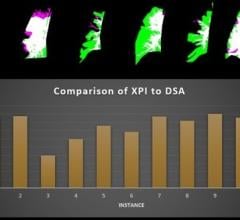
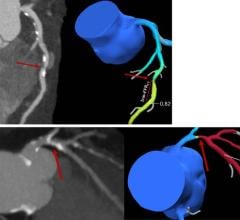
The qRA workflow provides objective and reproducible quantification of aortic regurgitation by using density of contrast in the aortic root and ventricle based on X-ray aortogram images. Visual determination is inaccurate and can lead to underestimation of regurgitation. The qRA workflow is developed to quantify the regurgitation directly after percutaneous valve replacement. In addition to the qRA workflow, CAAS A-Valve assists to define the optimal C-arm projection to place the prosthetic valve.
“The qRA workflow is the first technology to enable an objective and reproducible method for grading aortic regurgitation on contrast aortography and can be used in clinical practice or research,” said C. Schultz, M.D., Royal Perth Hospital, The University of Western Australia.
For more information: www.piemedicalimaging.com


 August 14, 2025
August 14, 2025