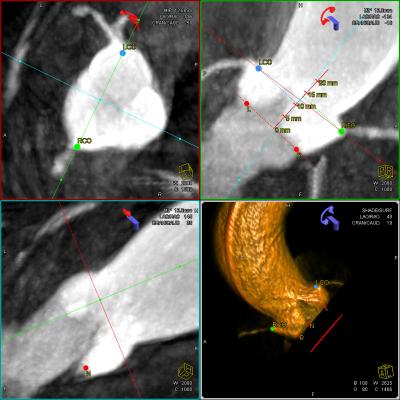
February 16, 2012 — Siemens Healthcare received clearance from the U.S. Food and Drug Administration (FDA) for syngo Aortic ValveGuide, an integrated image processing software that helps cardiologists and cardiac surgeons prepare and perform transcatheter aortic valve replacement (TAVR). The syngo Aortic ValveGuide automatically reconstructs a 3-D representation of the aortic root from cross-sectional images acquired with the angiography system and provides the best projection angle for the valve replacement. The software selects anatomical landmarks and overlays the 3-D image with 2-D images acquired during live fluoroscopy, enabling the physician to obtain real-time 3-D guidance in the patient’s body while navigating the new valve to its intended location. This groundbreaking software, which is now available to customers, reflects the spirit of Siemens Healthcare’s Agenda 2013 – a recently announced two-year initiative designed in part to strengthen the Healthcare Sector's innovative power and competitiveness.
During the minimally invasive TAVR intervention, an artificial aortic valve is inserted via the femoral artery or through the apex of the heart. syngo Aortic ValveGuide provides the physician with automated 3-D guidance by segmenting the aortic root in 3-D mode from images created using the angiography system. With the aid of anatomical landmarks in the 3-D vessel representation, syngo Aortic ValveGuide calculates the exact perpendicular view on the aortic root. The C-arm adjusts to the corresponding angulations for live fluoroscopy, enabling the physician to precisely position the new valve. Consequently, as soon as the software overlays the 3-D image of the aorta with the 2-D live fluoroscopy, the cath lab cardiologist – or, respectively, the heart surgeon in the hybrid OR – can begin the intervention. Since syngo Aortic ValveGuide only requires a short fluoroscopy time prior to the procedure, the patient's exposure to radiation and contrast can be reduced considerably.
Prior to syngo Aortic ValveGuide, users had to rely on preprocedural (or intraprocedural) CT, manually co-registering it with live fluoroscopy and overlaying it on a live fluoroscopy image to determine the correct angulation. This process required additional steps and highly skilled personnel. The new syngo Aortic ValveGuide uses syngo DynaCT Cardiac – Siemens software that processes CT-like cardiac images from images acquired using the angiography system.
For more information: www.siemens.com/healthcare


 August 14, 2025
August 14, 2025 









