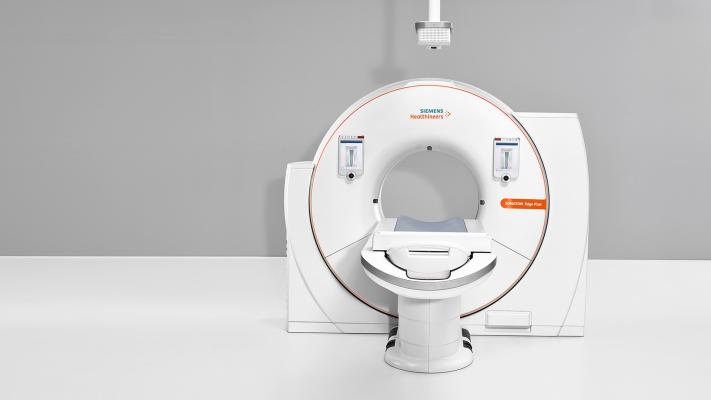
April 4, 2018 — The U.S. Food and Drug Administration (FDA) has cleared the Somatom Edge Plus computed tomography (CT) system from Siemens Healthineers with the FAST (Fully Assisting Scanner Technologies) Integrated Workflow with the FAST 3D Camera. The camera is the first patient positioning system powered by artificial intelligence (AI). Additionally, the new scanner allows for automated scan preparation, improved diagnostic confidence and customized patient dose, making it ideal for high-volume practices and emergency imaging environments.
Incorrect patient positioning in CT may lead to additional image noise or increased levels of patient radiation dose. The FAST Integrated Workflow and FAST 3D Camera of the Somatom Edge Plus enables accurate, reproducible patient positioning. Fixed above the patient table, the FAST 3D Camera uses infrared technology and AI to recognize anatomical landmarks. The table then aligns to the correct position to scan the body region at isocenter based on the selected protocol. This means institutions can streamline image quality, reduce dose and potentially reduce repeat scans.
Equipped with TwinBeam Dual Energy, the 128-slice Somatom Edge Plus enables quantitative imaging and improved tissue characterization for diagnostic confidence. This additional information allows for precise visualization of iodine uptake in a tumor. The system’s split filter technology permits acquisition of two spectral energies for dose-neutral Dual Energy scans in clinical routine. Using this technology, providers may be able to reduce additional non-contrast scans by producing virtual non-contrast images based on the Dual Energy data.
The Somatom Edge Plus enables scanning of obese patients with strong diagnostic confidence due to its Straton MX Sigma X-ray tube, high power reserves at every kV value in 10-kV increments and the Stellar Infinity detector – resulting in sharp, high-contrast images at high speed and low dose. Tin Filter technology, which can be used in all routine examinations, enables CT scans at very low doses by shielding patients from clinically irrelevant radiation. In the case of CT lung scanning, for example, this technology enables scanning at levels associated with a standard X-ray examination.
For more information: www.usa.siemens.com


 March 03, 2026
March 03, 2026 









