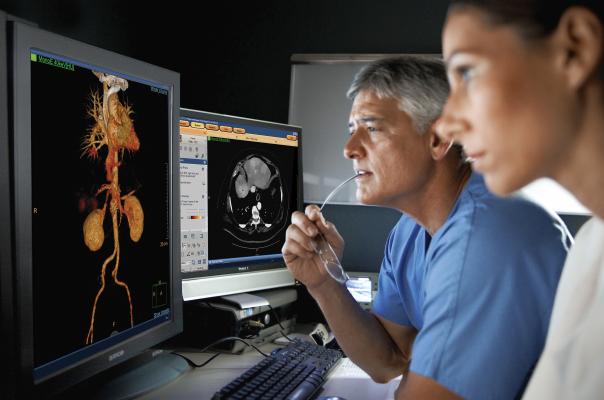
Radiologists using the Philips spectral CT software to view a spectral CT image reconstruction at various energy levels.
September 9, 2015 — The U.S. Food and Drug Administration (FDA) granted 510(k) for Philips Healthcare’s Spectral Diagnostic Suite (SpDS), which offers a set of advanced visualization and analysis tools designed for the IQon Spectral computed tomography (CT) technology. The software offers enhanced spectral viewing and clinical applications capabilities.
CT imaging is widely used in the diagnosis of many different diseases and injuries. By providing spectral capabilities within traditional CT applications, SpDS offers a new level of flexibility for CT users. It allows clinicians to utilize the spectral information on-demand, without the added complexity of special modes or workstations that disrupt user workflow. Additionally, because there is no need to bring the patient back for additional imaging, on-demand spectral analysis of a particular region allows the physician to further analyze incidental findings.
"Philips Spectral suite offers a new level of control for clinicians, allowing for segmentation on different energy levels, more detailed comparison between images, and advanced fusion capabilities, for diagnostic confidence,” said Prabhakar Rajiah, MBBS, M.D., FRCR, assistant professor of radiology, University Hospitals of Cleveland, UH Case Medical Center, Department of Radiology, who collaborated with Philips on the development of the suite.
The Philips SpDS package includes:
· Spectral enhanced Comprehensive Cardiac Analysis (sCCA)
· Spectral enhanced Advanced Vessel Analysis (sAVA)
· Spectral enhanced Tumor Tracking (sTT).
The Philips SpDS also allows for viewing and analysis of spectral datasets in a variety of settings: a reading room, on picture archiving and communication systems (PACS), or from remote locations.
Spectral CT imaging allows viewing of the same anatomy at two different kV energies. Different anatomical features are enhanced and easier to see at different energy levels. Additionally, the spectral software can highlight or eliminate specific chemical compounds based on their atomic number, including iodine, calcium, etc. This allows differentiation between calcified coronary lesions and iodine contrast in the blood vessel, or enables contrast and non-contrast images from the same scan.
For more information: www.spectralctlearningcenter.philips.com


 March 06, 2026
March 06, 2026 









