April 7, 2009 - The FDA today gave marketing clearance to a new product from Carestream Health: The CARESTREAM DRX-1 System, a wireless digital radiography (DR) detector—the size of a standard cassette—that fits existing X-ray rooms without having to replace or modify existing equipment.
With this clearance, Carestream Health can begin taking orders for the product. Shipping will begin in the next several weeks, following trade trials and other advance preparations that are underway.
The CARESTREAM DRX-1 System incorporates a console and a wireless 14 x 17 inch (35 x 43 cm) cassette-size DR detector that provides a rapid, affordable conversion for users of radiographic film or computed radiography (CR) systems. Since the system requires no modifications to existing film-based equipment, installation costs are modest—and healthcare facilities can use one detector for nearly all types of exams where a traditional cassette would be used. The DRX-1 system delivers high-quality preview images in about five seconds, which significantly improves productivity, even for users of computed radiography (CR) systems.
“This innovative wireless detector presents an extremely attractive option for facilities that want to improve productivity and image quality in existing film or CR rooms, but do not have the budget for equipment replacement,” said Diana L. Nole, President, Digital Medical Solutions, Carestream Health. “No modifications to existing X-ray systems are needed and facilities can continue to use the Bucky with CR or film-based cassettes if desired. In addition, the wireless functionality of the DRX-1 can improve efficiency by allowing a much more flexible workflow to meet the specialized needs of each individual facility.”
Because this DR detector is a wireless cassette, it provides flexible positioning that enhances both efficiency and patient comfort. The detector can be used wherever it is needed—in the wall stand Bucky, table Bucky or for tabletop shots and other difficult views.
With a weight of 8.5 pounds, the detector is up to 30 percent lighter and up to 50 percent smaller than other portable detectors. Its compact size and light weight further enhance convenience and throughput for radiology professionals.
Carestream Health’s engineers designed the DR detector and all components within the cassette to withstand the challenging environment of a modern X-ray department. The rugged detector, case, and internal components are designed to produce top quality DR images under real-life X-ray department conditions.
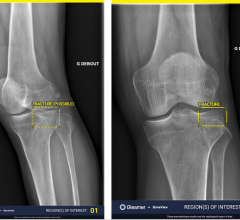
The DRX-1 system—suitable for general radiology, trauma, orthopedics and virtually all other X-ray exams—incorporates the same innovative software and image processing capability as Carestream Health's KODAK DirectView CR and DR systems and, therefore, will deliver image quality and workflow consistent with these systems.
A console is included with the detector to assist with image capture, preparing preview images, image processing and full-resolution display. Images can be transmitted as DICOM files to a PACS or storage device.
The DR detector can be used with standard off-the-shelf grid holders and grids for tabletop use.
For more information: www.carestreamhealth.com


 February 10, 2026
February 10, 2026