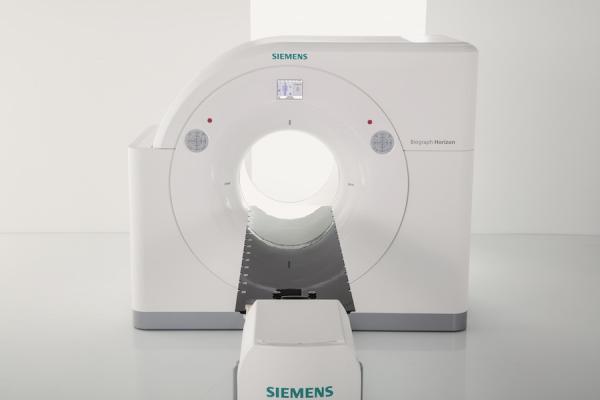
Siemens Healthineers announced that the Food and Drug Administration (FDA) has cleared Biograph Horizon Flow edition, a cost-competitive positron emission tomography/computed tomography (PET/CT) system that features the company’s revolutionary FlowMotion continuous bed motion scanning technology. With products such as the Biograph Horizon Flow edition, as well as a new name that underlines the company’s pioneering spirit and engineering expertise, Siemens Healthineers – the separately managed healthcare business of Siemens AG – is helping to enable healthcare providers worldwide to meet current challenges and excel in their respective environments.
Initially introduced on the company’s premium Biograph mCT Flow PET/CT platform in 2013, FlowMotion continuous bed motion technology differs from conventional step-and-shoot PET/CT scanning in that it enables personalized exam protocols based on patient anatomy. These protocols can be configured based on the radiology department’s most commonly scanned indications to support standardization. With the extension of FlowMotion continuous bed motion technology to Biograph Horizon Flow edition scanners, users can define up to four distinct scanning regions, each with a different bed speed. Additionally, specific exam reconstructions can be applied to each scanning region or to multiple regions. Biograph Horizon Flow edition facilitates high-resolution reconstructions and respiratory gating, which can help improve the ability of clinicians to detect lesions.
In addition to FlowMotion continuous bed motion technology, Biograph Horizon Flow edition includes HD•Chest – another popular feature previously found only on the company’s Biograph mCT family of PET/CT systems – which acquires and processes motion-frozen chest scans five times faster than standard respiratory gating techniques.
“With the addition of Biograph Horizon Flow edition to the Siemens Healthineers PET/CT portfolio, customers tasked with reaching a certain price point no longer need to make compromises in the PET/CT technology they offer patients,” said Doug Darrow, Vice President of Molecular Imaging at Siemens Healthineers North America. ‘The Biograph Horizon Flow edition is the ideal https://www.itnonline.com/channel/pet-ct not only for progressive community hospitals but also for satellite campuses of large healthcare networks that are looking to provide the same premium continuous bed motion technology as their flagship facilities.”
For more information: www.siemens.com/healthineers


 January 22, 2026
January 22, 2026