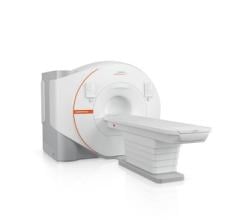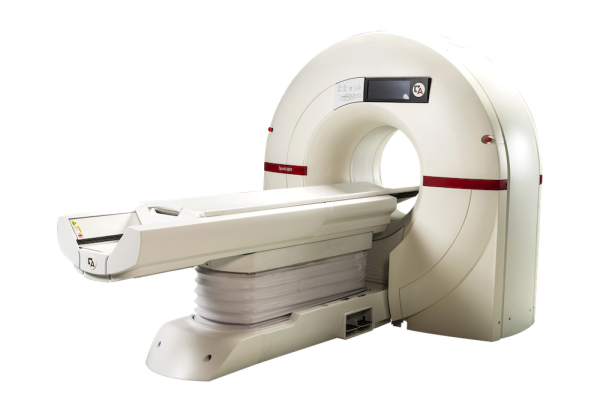
May 19, 2025 - Arineta, a provider of cardiovascular imaging solutions, recently announced that its SpotLight Duo cardiac CT scanner has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for low-dose lung cancer screening (LDCT). This clearance allows providers to use a single ultra-fast CT platform for both cardiac and lung imaging and diagnostics, delivering more complete care to high-risk patients.
Lung cancer is the leading cause of cancer deaths in the U.S., with a new patient diagnosed nearly every two minutes. However, today, more people are surviving lung cancer than ever before, and over the past five years, the survival rate has increased by 26% nationally to 28.4%. Arineta’s FDA clearance expands access to the critical, lifesaving imaging needed for this high-risk population.
“This clearance marks a significant milestone in expanding our role in early detection and preventative care,” said Doug Ryan, CEO of Arineta. “We are dedicated to growing cardio-thoracic CT as the front-line non-invasive test for diagnosing, therapy planning, and monitoring of cardiovascular and thoracic disease. The addition of FDA clearance for Low Dose Lung Cancer CT strengthens our ability to support improved outcomes for patients at high risk of disease.”
As awareness grows around preventative imaging and population health strategies, this clearance expands the clinical utility of Arineta’s SpotLight Duo across health systems, provider offices, mobile scanning units, and imaging centers nationwide. With the addition of this clinical application for LDCT, the system supports earlier detection and more proactive care for patients.
The Spotlight Duo cardiac CT scanner captures the entire heart in a single beat, featuring 140 mm coverage at a rotation speed of 0.24 sec per rotation. Its advanced deep-learning imaging reconstruction (DLIR) technology enhances image quality by reconstructing cross-sectional images, resulting in more comprehensive and accurate diagnoses.
For more information, visit arineta.com.


 February 13, 2026
February 13, 2026