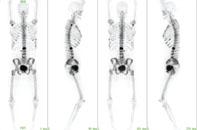
February 8, 2011 – In an announcement by the National Cancer Institute (NCI) and the National Institutes of Health (NIH), the U.S. Food and Drug Administration (FDA) has approved a New Drug Application (NDA) for use of a new strength of a previously approved drug, sodium fluoride F 18 (18F-NaF) injection for use in bone scans.
Petnet Solutions Inc., a wholly owned subsidiary of Siemens Medical Solutions USA Inc., is the only commercial manufacturer to be included in this NDA for 18F-NaF injection, at this time.
For more information: www.siemens.com/healthcare


 March 09, 2026
March 09, 2026 









