Image courtesy of ECRI
December 4, 2014 — To help hospitals reduce technology-related risks, ECRI Institute publishes an annual list of Top 10 Health Technology Hazards.
The 2015 hazards list highlights 10 safety topics and details a variety of technology hazards that put patients at risk.
Each hazard includes an overview of the issue and recommended action steps to aid healthcare facilities in their efforts to maintain a safe environment for patients and healthcare workers. Topics include:
1. Alarm hazards: Inadequate alarm configuration policies and practices
2. Data integrity: Incorrect or missing data in electronic health records and other health IT systems
3. Mix-up of IV lines leading to misadministration of drugs and solutions
4. Inadequate reprocessing of endoscopes and surgical instruments
5. Ventilator disconnections not caught because of mis-set or missed alarms
6. Patient-handling device use errors and device failures
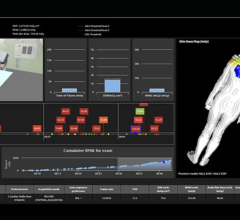
7. “Dose creep”: Unnoticed variations in diagnostic radiation exposures
8. Robotic surgery: Complications due to insufficient training
9. Cybersecurity: Insufficient protections for medical devices and systems
10. Overwhelmed recall and safety alert management programs
This year, the report draws particular attention to alarm configuration practices. ECRI Institute is aware of several deaths and other cases of severe patient harm that may have been prevented with more effective alarm policies and practices.
Recall management points to overwhelmed recall and safety-alert programs as a potential for serious consequences for healthcare facilities and patients. ECRI experts are concerned that existing hospital recall tracking programs are not keeping pace with the growing number of medical device recalls issued each year. The U.S. Food and Drug Administration (FDA) reports that the annual number of medical device recalls nearly doubled between 2003 and 2012, from 604 recalls to 1,190 annually.
For each topic, ECRI Institute describes the hazard, presents recommendations for minimizing the risks, and lists helpful resources that readers can access to learn more about the topic.
To develop the annual list, ECRI Institute’s multidisciplinary staff of engineers, scientists, nurses, physicians and patient safety analysts draw on the resources of the Institute’s 45-year history, as well as expertise and insight gained through testing and analyzing healthcare technologies. This includes examining health technology-related problem reports from hospitals and health systems worldwide, and reports received through ECRI Institute Patient Safety Organization.
For more information: www.ecri.org


 February 04, 2026
February 04, 2026