March 13, 2012 – Fischer Medical Technologies LLC (Fisher Medical), announces from the National Interdisciplinary Breast Center Conference that it has been named exclusive U.S. distributor of GIOTTO’s Full Field Digital Mammography (FFDM) systems.
After receiving 510(k) clearance by the FDA in November 2011 for the GIOTTO FFDM system, I.M.S. Srl (Internazionale Medico Scientifica) of Bologna Italy and Fischer Medical of Broomfield, Colo. signed a multiyear distribution agreement. The newly-formed Fischer-GIOTTO division is headed by Bob Rusk, who formerly managed GIOTTO-USA.
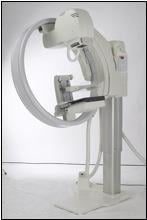
The unique design of the GIOTTO system makes it possible to dedicate a single room for both upright mammography, as well as prone stereotactic biopsy. The innovative tilting gantry ensures optimal positioning so that more breast tissue is included in the imaging field, increasing the ability to detect small cancers on the chest wall. It also enables the mammogram procedure to be done “face to face;” a benefit to radiologists, technologists and patients alike. The ring design of the GIOTTO system allows easy positioning of disabled wheelchair patients – the only known mammography system with this capability.
Morgan W. Nields, chairman and CEO of Fischer Medical said, “We are delighted with this agreement since the GIOTTO FFDM system has been installed in over 500 sites around the world and has demonstrated a proven track record of superb image quality and reliability.”
Vice President of International Sales at IMS in Bologna, Achille Alabanse said, “We have been gaining market share throughout the world and are very pleased to join forces with Fischer Medical. This agreement leverages Fischer’s deep knowledge and installed base of prone biopsy systems in the United States.”
For more information: www.giottousa.com


 February 18, 2026
February 18, 2026 









