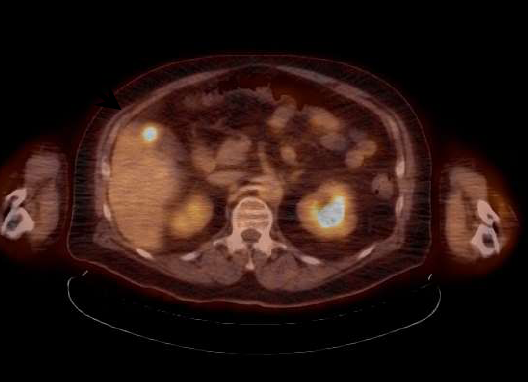
June 23, 2016 — Cancer patients often experience significant fluctuations in weight and lean body mass (LBM). Neglecting to account for these changes can prevent clinicians from obtaining precise data from molecular imaging, but a new method of measuring LBM takes changes in individual body composition into account for better staging of disease and therapy monitoring, said researchers at the 2016 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI), June 11-15 in San Diego.
Positron emission tomography (PET) has become a standard of care in cancer patient management. Fluorine-18 fluorodeoxyglucose (F-18 FDG) is a radiotracer that closely resembles the metabolic activity of glucose in cells throughout the body. F-18 FDG emits a signal that is detected by the PET scanner. Image reconstructions of these signals let clinicians know where there are areas of abnormally increased metabolic activity, quantified by standardized uptake values (SUV). Accurate tumor SUVs enable clinicians to assess disease progression, reflected by an increased SUV; stable disease, reflected by no significant changes in SUV; and successful response to therapy, reflected by a decrease in SUV.
Radiotracer tumor SUVs are quantitatively measured using patient weight. However, F-18 FDG is distributed almost exclusively in LBM, not fatty tissue. Current methods of SUV normalization tend to focus on overall weight and may significantly over- or underestimate LBM in cancer patients. For this study, researchers developed a computed tomography (CT) technique that determines patient-specific LBM. This method of SUV normalization, using patient-specific LBM, is termed SULps.
“Patients with advanced cancer tend to lose muscle and may gain fat, and these changes in body composition can significantly modify PET results, independent of the actual metabolic activity of the tumor,” said Alexander McEwan, M.D., principal author of the study from the University of Alberta Edmonton in Alberta, Canada. “Our study shows that CT-derived SULps is a more robust measurement for patients with advanced cancer undergoing PET imaging. If adopted, this simple change in imaging protocol could lead to significantly more effective care for cancer patients.”
Researchers analyzed CT scans in three-month intervals to gauge changes in body composition of patients with advanced cancer. A total of 1,080 intervals of CT were evaluated and just over 50 percent, or 546 intervals, reflected stable LBM. Of this smaller group with stable LBM, 40 percent experienced a decrease in adipose tissue, 35 percent showed no change in adipose tissue, and 25 percent showed an increase in adipose tissue. These changes affect SUV using all calculations except SULps, which accurately reflected metabolic activity via F-18 FDG PET/CT.
More precise PET measurements made with the use of CT data, could increase the accuracy of PET imaging interpretation in ground-breaking clinical trials for new cancer therapies and for more personalized medicine in the clinic.
For more information: www.snmmi.org


 February 11, 2026
February 11, 2026