July 1, 2019 — Bracco Imaging S.p.A. has signed a definitive agreement to acquire Blue Earth Diagnostics, a molecular imaging company based in Oxford, U.K. Subject to customary closing conditions, completion of the transaction is expected in the third quarter of this calendar year.
Bracco Imaging will acquire all outstanding shares of privately-held Blue Earth Diagnostics for the equity value of $450 million, plus closing adjustment estimated at $25 million, from healthcare company Syncona and Blue Earth Diagnostics' management team. Upon closing of the transaction, Blue Earth Diagnostics will be a subsidiary of Bracco Imaging, led by its current leadership team and will retain the Blue Earth Diagnostics name. Blue Earth Diagnostics employs approximately 100 people and is expected to generate revenues of $140 million in the year to September 2019, primarily in the U.S.
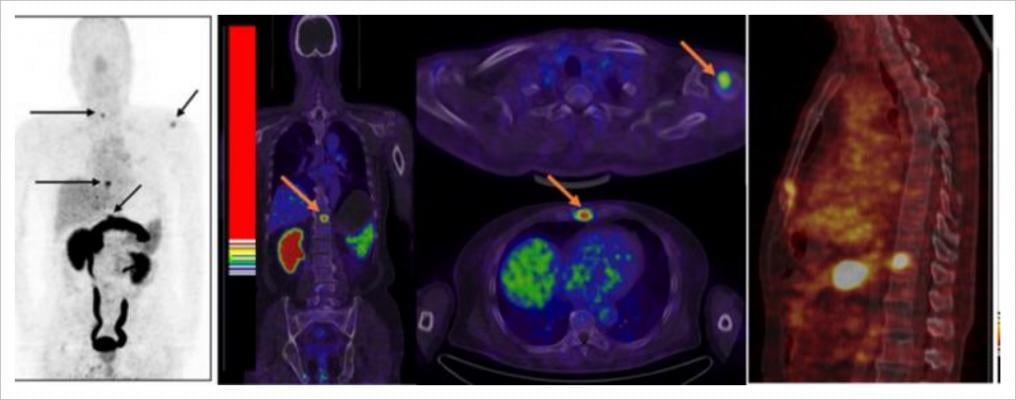
The first novel positron emission tomography (PET) molecular imaging agent developed by Blue Earth Diagnostics is Axumin (F18-fluciclovine) injection. Axumin is approved in the U.S. and the European Union for PET imaging in men with suspected recurrent prostate cancer based on elevated prostate specific antigen (PSA) levels following prior treatment. The company's pipeline includes prostate specific membrane antigen (PSMA)-targeted radiohybrid ("rh") agents, which are a clinical-stage, investigational class of theranostic compounds, with potential applications in both the imaging and treatment of prostate cancer.
Prostate cancer is the most common cancer in men, with an estimated number of more than 170 thousand new cases in 2019 in the U.S. While most cases of primary prostate cancer can be treated successfully, the cancer will recur in up to one third of cases and in a third of those recurrences, patients develop distant metastases leading to a fatal outcome. In two separate studies which evaluated the utility of Axumin (F18-fluciclovine) PET/CT (computed tomography) in providing physicians with actionable information for the management of men with recurrent prostate cancer, the intended management plan was changed for approximately 60 percent of the study subjects, based on the results of the Axumin PET/CT scan.
F18-fluciclovine has a broad range of other potential applications in cancer imaging and Blue Earth Diagnostics is investigating the molecule for other cancers including in neuro-oncology.
For more information: www.braccoimaging.com, www.blueearthdiagnostics.com


 February 13, 2026
February 13, 2026 









