January 27, 2016 — Effective Jan. 1, 2016, the American Medical Association (AMA) Current Procedural Terminology (CPT) Editorial Panel assigned a new category III CPT code, 0399T, for use in reporting myocardial strain imaging for the detection of myocardial deformation. While Category III codes generally are not initially reimbursed, they often become payable when they are used routinely in clinical practice.
Research has shown that strain imaging can improve diagnostic assessment, standardize interpretation and assist in improving the monitoring of patients over time to optimize care. Additionally, measurements derived from strain imaging have been shown to be more sensitive and less variable than gold standard measurements like EF, making it increasingly important.
American Society of Echocardiography (ASE) chamber quantification guidelines now recommend strain imaging when assessing function. ASE recommendations for assessment and monitoring with echo of cardio-oncology patients indicate strain imaging should be monitored in this patient population.
The ACVI/ASE/Industry Task Force to Standardize Deformation Imaging issued definitions to improve standardization of 2-D speckle tracking measurements.
Increasingly, studies show that assessing and monitoring strain may improve quality of patient care and reduce costs with patient readmissions.
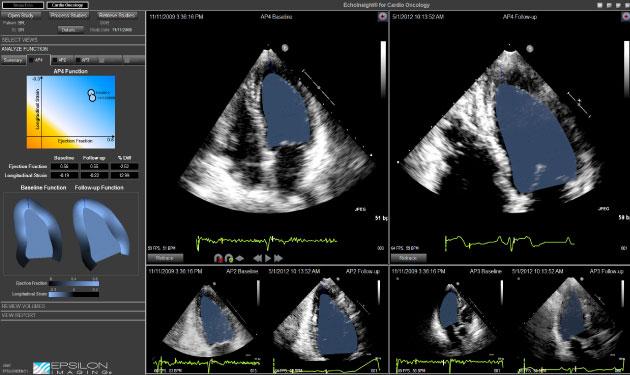
Although strain imaging has proven to be valuable in the assessment and management of patients with echo, until now, diagnostic software solutions with strain imaging have been research-oriented, laborious and workflow-inefficient in clinical practice.
EchoInsight is among the companies pushing for increased usage of strain imaging to improve diagnostic confidence, standardization and efficiency. Epsilon Imaging’s proprietary speckle tracking technology, TissueTrack, provides robust strain imaging with automation of cardiac functional measurements. It provides clinical strain imaging for improved confidence in assessment and monitoring of wall mechanics for the entire heart. It is available as a stand-alone workstation or client-server architecture using a vendor neutral platform.
For more information: www.epsilon-imaging.com


 February 05, 2026
February 05, 2026 









