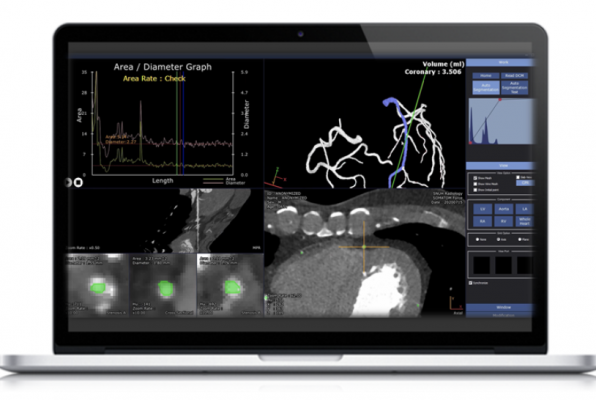
November 30, 2020 — AI Medic Inc. announced that it has obtained official product certification from the NIDS (National Institute of Medical Device Safety Information) for its own medical video analysis software called "AutoSEG". "AutoSEG" is the only medical device in the world that automatically shapes a highly difficult coronary artery in three dimensions without expert intervention, assisting the diagnosis of diseases such as heart angina and myocardial infarction.
AutoSEG, which has been certified as a product by the NIDS, is a medical device that can automatically extract the coronary artery, aorta, ventricle and atrial components of the heart from CT images through the fusion of AI (Artificial Intelligence) techniques and numerical algorithms, and shape them in three dimensions and accurately measure value.
The heart is the only organ that continues to exercise during CT scans, and measurement is very difficult. Previously released commercial software takes about 4 to 8 hours, even if experts do it themselves, using semi-automation that allows users to extract areas by hand. On the other hand, AI Medic's AutoSEG allows three-dimensional segmentation of heart components from heart CT images within 10 minutes without the help of experts.
In addition, AI Medic is currently developing HeartMedi, which implements non-invasive CT-FFR technology that analyzes fractional flow reserve (FFR) by combining corresponding Auto Segmentation technology with hemodynamic technology. The medical device, which can determine whether a coronary artery is diseased through a hemodynamic simulation, is currently in the final stage of clinical trials in Korea. CT-FFR is an innovative technology that can predict FFR with CT images only, and HeartMedi is a medical imaging assist software that implements this technology. In the case of conventional invasive methods, not only did the patient suffer pain and danger, such as injecting guide wire, pressure sensor, and drugs into the blood vessels, but HeartMedi from AI Medic is receiving market attention as it can overcome all of these limitations. The success of these technologies is expected to drastically reduce unnecessary stent procedures, and also help the health insurance budget's worsening health care system.
Based on this product certification, AI Medic is focusing its efforts on marketing 'AutoSEG' while successfully carrying out clinical trials of HeartMedi, and aims to supply products for diagnosing heart diseases in the future starting from this year, starting with the supply of research version products mainly at major university hospitals.
Meanwhile, AI Medic is a company established in 2014 and is a startup that analyzes the physiological phenomenon of the human body through AI and computer simulation and develops a technology to diagnose patient-specific cardiovascular and cerebrovascular diseases based on CT-FFR, a core technology of cardiovascular digital healthcare, and medical imaging technology. It has completed investments of 3 billion won from Magna Investment in May 2018 and 7 billion won from Intervest in late March this year.
For more information: http://www.aimedic.kr/


 February 06, 2026
February 06, 2026 









